Citation: Han L, Ji Y, Yu Y, Ni Y, Zeng H, Zhang X, et al. (2024) Trajectory-centric framework TrajAtlas reveals multi-scale differentiation heterogeneity among cells, genes, and gene modules in osteogenesis. PLoS Genet 20(10):
e1011319.
https://doi.org/10.1371/journal.pgen.1011319
Editor: Christine Wells, University of Melbourne, AUSTRALIA
Received: May 28, 2024; Accepted: October 7, 2024; Published: October 22, 2024
Copyright: © 2024 Han et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The Differentiation Atlas (raw counts, embedding, cell type annotations, biological and technical metadata) is publicly available and can be downloaded via Figshare (https://figshare.com/articles/dataset/Differential_Atlas/25422688). The annotation system and OPC mapping of Differentiation Atlas can be interactively explored at https://zyflab.shinyapps.io/TrajAtlas_shiny/. The TrajAtlas software package is available at https://github.com/GilbertHan1011/TrajAtlas. The TrajAtlas documentation including API, tutorials, and examples is available at https://trajatlas.readthedocs.io/. Codes to reproduce our analysis are available at https://github.com/GilbertHan1011/TrajAtlasManuscript
Funding: This work was funded by grants from the National Key Research & Development Program of China (2021YFC2400405) to YZ; the National Natural Science Foundation of China (No. 82322014 and No. 82270948) and The Interdisciplinary Research Project of School of Stomatology Wuhan University (No. XNJC202306) and the Fundamental Research Funds for the Central Universities (2042022dx0003, 2042024kf1023) to HL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Osteoblasts, or bone-forming cells, play a critical role in the dynamic processes of bone formation [1,2], remodeling [2,3], and regeneration [4]. Due to their limited lifespan, osteoblasts require constant replenishment by osteoprogenitor cells (OPCs) [5]. This process, known as osteoblast differentiation [5], occurs in various tissues and different ages in response to various stimuli such as development and regeneration [5]. Across these tissues and age groups, diverse osteoprogenitors contributing to this process have been identified [5,6]. In this respect, recent studies have sequentially identified bone marrow stromal cells expressing markers Lepr [5–7], Grem1 [8], and Cdh2 [9] as osteoprogenitors in long bone. Besides, chondrocytes, expressing Pthlh [5,6,10], Foxa2 [11], and Fgfr3 [12], were also identified as cellular sources of osteoblast in long bone. The diversity of osteoprogenitor cells necessitates a systematic classification. However, the current approach based on immunophenotypic markers may be too crude to fully capture the big picture of osteoprogenitors [5,6].
The crude definition of osteoprogenitor cells has largely hindered our understanding of osteoblast differentiation. Multiple studies have proposed distinct pathways for osteogenesis in long bones, resulting in a fragmented view of the process [5,6,10,13,14]. While efforts to synthesize these findings into a unified framework have been undertaken [15], they failed to illuminate the intricacies of osteoblast differentiation. The current classification system, which dichotomizes osteogenesis into endochondral and intramembranous based on the presence of a cartilaginous template [16], overlooks the diverse cellular origins of osteoblasts. This oversimplification emphasizes the need for a novel model that incorporates the origin of osteoblasts, thereby providing a more accurate representation of osteoblast differentiation and addressing the existing knowledge gaps.
While cell origin may influence the differentiation process, there are many other covariates to consider, such as age [17,18], tissue [5,6], and injury [7], which may also contribute to heterogeneity. For example, studies have shown that the expression of certain genes, such as Maf, declines with age [17]. Conversely, under injury conditions in long bones, the Wnt signaling pathway is upregulated to promote bone regeneration [7]. The influence can occur in different aspects, including gene expression [19], transcription factors [20], and pathways [20], and these changes may vary throughout the differentiation process. For example, the early stages may differ significantly from the later stages [21]. These factors render differentiation a complex puzzle and make it difficult to fully grasp the concept.
In recent years, single-cell technologies have revealed cellular states with unprecedented detail, providing insights that facilitate the reconstruction of differentiation processes. Numerous algorithms have emerged, each aiming to reconstruct differentiation from different perspectives [22,23]. However, these methods are often isolated and lack a comprehensive view of differentiation. Each method raises distinct questions—such as the sequence and fate of cells during differentiation (Monocles [22], Slingshot [23]), the changes in gene expression patterns over time (tradeSeq [19]), and the influence of covariates on this process (Lamian [21], Condiments [19]). However, to fully understand the dynamics of differentiation, it is essential to examine cells, genes, and gene modules across the diverse spectrum of differentiation trajectories. This limitation underscores the need for more sophisticated analytical frameworks that can integrate and interpret the vast and intricate data generated by single-cell technologies, ultimately providing a more nuanced and holistic understanding of cellular differentiation processes.
To address the complexity and heterogeneity inherent in osteoblast differentiation, our study introduces TrajAtlas, a trajectory-centric framework specifically designed to navigate and elucidate these challenges. TrajAtlas encompasses a reference atlas for osteoblast differentiation coupled with a sophisticated seven-layer hierarchical annotation system that allows for the exploration of osteoprogenitor cell heterogeneity. In-depth, a model was introduced to reveal genetic regulation and differentiation pathways from diverse osteoprogenitor cells to mature osteoblasts. It features advanced tools for dissecting cell and gene dynamics along trajectories, facilitating the identification of age and tissue-specific variations and uncovering differentiation-related gene modules to infer their activity within these paths. By leveraging TrajAtlas, with a particular focus on the influences of age and injury on bone formation, our framework unveils novel insights into osteoblast differentiation. Importantly, while our framework is exemplified through the study of osteogenesis, it is designed to be broadly applicable. When supplemented with single-cell transcriptome datasets from other tissues or lineages, TrajAtlas can be adapted to reveal similar insights into the differentiation processes of various cell types, making it a versatile tool for understanding cellular differentiation across different biological contexts. We have made the associated datasets available for interactive exploration at https://zyflab.shinyapps.io/TrajAtlas_shiny, and the corresponding computational framework as open-source software, available at https://trajatlas.readthedocs.io.
Results
Overview of TrajAtlas
Cell differentiation is a complex problem involving changes in cells, genes, and gene modules. To address this complexity in a more comprehensive way, TrajAtlas comprises four distinct modules, enabling the multi-scale exploration of differentiation heterogeneity. (a) Heterogeneity of Osteoprogenitor Cells. The first module, the Differentiation Atlas, integrates trajectories across diverse tissues and ages to create a comprehensive reference atlas of osteoblast differentiation, covering 110 samples and 272,369 cells (S1 Table). A seven-level hierarchical annotation system reconciles conflicting osteogenesis-related cell types across different studies. By mapping experimentally validated osteoprogenitor cells onto this atlas, we can more thoroughly investigate their heterogeneity (Fig 1A). (b) Genetic Regulation and Pathways Driving Osteoprogenitor Differentiation: the second module, the Differentiation Model, reconstructs the osteoblast differentiation process. It employs four trajectory groups to represent the differentiation pathways from various osteoprogenitor cell populations, offering insights into the critical signaling pathways and gene regulatory networks that govern this process (Fig 1B). (c) Age and Tissue-Specific Variations in Differentiation: the third module, TrajDiff, facilitates the detection of covariate-associated differential cell abundance and gene expression along the differentiation process across multiple trajectories. This tool allows for the assessment of factors such as age and tissue-specific location on gene expression patterns during osteoblast differentiation (Fig 1C). (d) Identification of Gene Modules Related to Differentiation: the fourth module, TRAVMap, introduces novel methods for identifying robust gene modules across multiple datasets. It enables the investigation of the roles these gene modules play within large-scale differentiation trajectories and their relationship to differentiation processes (Fig 1D). Through these approaches, we enable the unraveling of differentiation heterogeneity in cells, genes, and gene modules.
Fig 1. Overview of TrajAtlas: A framework designed to unravel osteogenesis heterogeneity in a multi-scale manner.
(A) Differentiation Atlas integrates trajectories spanning various tissues and continuous age groups to construct a differentiation atlas aimed at identifying various osteogenic precursor cells (OPCs). (B) Differentiation Model reconstructs the osteoblast differentiation process, unveiling key genes and transcription factors associated with OsteoProgenitor Cell-Specific Trajectory (OPCST). (C) TrajDiff detects covariates-related differential cell abundance and gene expression along the differentiation process across multiple trajectories. (D) TRAVMap module identifies trajectory-related gene modules and infers gene module activity across large-scale trajectories. This figure was created with BioRender.com.
The osteogenic differentiation atlas reveals the heterogeneity of osteoprogenitor cells
To construct a comprehensive map of osteoblast differentiation, we combined 26 public datasets into the Differentiation Atlas, encompassing a total of 272,369 cells (Fig 2A). These datasets originated from three primary osteogenic tissues (head, limb bud, and long bone [5,6]) across various age groups, ranging from embryo to old age (Methods). With a focus on the differentiation process, we filtered out cells irrelevant to osteogenesis (Methods). Next, we employed scANVI [24] to integrate the datasets, preserving biological variations while removing batch effects within the atlas (Figs 2A and S1A–S1D and S2 and Methods). Subsequently, we implemented a multi-level clustering method to visualize the hierarchical organization of cell populations (Figs 2C and S3 and Methods). We manually annotated the first three levels of clusters based on previous studies [5,6,25] and then utilized marker genes to distinguish finer cell states (Figs 2C and S4 and Note 1 in S1 Text). Furthermore, we harmonized findings from previous studies within the atlas and provided detailed descriptions of most clusters in the supporting information and online websites (S2 Table and Methods). With the extensive curated atlas and detailed annotations, we were able to explore the heterogeneity of osteoprogenitor cells.
Fig 2. Differentiation atlas reveals the heterogeneity of Osteoprogenitor cells.
(A) A schematic for differentiation atlas. (B) UMAP visualization of experimentally validated osteoprogenitors mapped to Differentiation Atlas. (C) A hierarchical tree of clusters of Differentiation Atlas. The first five levels with up to 49 clusters are presented, emphasizing the diverse nature of osteoprogenitors across various tissues and age groups. The left heatmap (red) depicts the overlapping of experimentally validated osteoprogenitors with clusters in the lowest tree level in the Differetiation Atlas. The middle heatmap (orange) depicts the relative percentage contribution of each cluster at the lowest tree level to the age group. The right heatmap (dark green) illustrates the relative percentage contribution of each cluster to the tissue origin group. In the right panel, dotplot displays marker genes at level 5 (Methods). (D) Barplots illustrates the proportions of cell types annotated with level-2 annotation across 65 samples with different tissue and age groups. Panel (A) and (D) were created with BioRender.com.
We found that osteoprogenitor cells exhibited diverse distributions [6] and expressed distinct characteristic markers (Fig 2C), as reported in previous studies [5,6]. We curated osteoprogenitor marker genes, tissue locations, ages, and validation methods from 28 studies on osteoprogenitors, the majority of which validate lineage tracing in vivo, providing the highest level of biological evidence (23 out of 28) (S3 Table). To assess the osteogenic potential of cells within our atlas, we incorporated experimentally validated osteoprogenitors based on previously described marker genes and tissue locations (Fig 2A). Notably, we observed that osteoprogenitor cells exhibit great diversity, and rather than being distinct isolated states, they may be interconnected (Fig 2A and 2B). Utilizing our level-2 annotation system, we categorized these osteoprogenitor cells into six major cell types: chondrocytes, mesenchymal cells (Mes), Ly6a+ Mes, LepR+ bone marrow stromal cells (LepR+ BMSCs), fibroblasts, and pericytes (Fig 2C).
Chondrocytes, a major cellular source of osteoblasts in the growth plate [26] were primarily located in the limb bud and long bone datasets within our atlas (Fig 2C and 2D). Chondrocytes can be further refined into four distinct clusters, all of which are reported to be osteoprogenitor cells with different spatial-temporal distributions. Chondrocyte progenitor cells (CPCs) typically express Grem1 [25] and Pthlh [27], which may located in the resting zone of the growth plate [5,27] (Figs 2C and S5B). Hmmr+ CPCs [10] represent proliferating osteoprogenitors in the articular cartilage and the growth plate (Figs 2C and S5A). Hypertrophic chondrocytes (HCs) [5,28] marked by Col10a1 can directly differentiate into osteoblasts (Fig 2C). Mature chondrocytes expressing Foxa2 [11] may contain long-term osteoprogenitors involved in growth plate regeneration (Figs 2C and S5C). Interestingly, the limb bud predominantly contained Hmmr+ CPCs, while the head exhibited a higher proportion of CPCs (Fig 2C). This distribution highlighted the potential influence of tissue origin and developmental stage on these subpopulations.
Mesenchymal cells (Mes), characterized by the expression of Prrx1 [29], are the main cellular source in embryo head and limb bud. They compose a large population in head and limb bud datasets in our atlas. As age increases, the cellular population of Mes declines and their cellular states undergo great variation [29] (Figs 2C and 2D and S6A and S6B). We annotated these states according to their prominent age as follows: Early Mes, predominant during the organogenesis stage (E8.5-E14), manifested the early perichondrial marker Hes1 [30] (Fig 2C); Middle Mes, the primary cell population in both organogenesis and fetal stage (E14.5-E18.5), specifically exhibited the suture mesenchyme marker Axin2 [31] (Figs 2C and S5D); Late Mes, the major cell population in the postnatal stage (P0-P30), highly expressed Msx2 [32] and may represent osteoprogenitors in specific craniofacial regions (Fig 2C). Besides, we found a cell state that highly expressed Ly6a (stem cell antigen-6), known markers of progenitor-like cells (S5E Fig). This population was notably present during the fetal stage in the head and limb regions (Fig 2D), as described in previous studies [29,33]. Consequently, we termed this cluster Ly6a+ Mes [33].
LepR+ BMSC cells, well-known for their roles in maintaining the bone marrow vasculature and regulating hematopoiesis [5–7], are multipotent cells residing within long bones [34]. Our atlas revealed LepR+ BMSCs in long bones only after the postnatal stage, with their population expanding as the organism ages (Fig 2D). Interestingly, Grem1+ BMSC [8] and Cdh2+ BMSC [9] identified in previous studies demonstrated significant overlap with LepR+ BMSC in our atlas (Fig 2B).
Fibroblasts, characterized by high expression of S100a4 [25], were primarily observed in the adult stage (3M-12M) of long bones (Fig 2D). We found that they expressed periosteum maker, such as Postn [35], indicating their potential as a source of osteoblasts (Figs 2C and S7L and S7M). Pericytes, characterized by high expression of Acta2 [25] (Fig 2C), constituted a minor population within our atlas (Fig 2D).
Our observations suggest that age is likely the most significant factor influencing the cellular states of osteoprogenitors (S6A and S6B Fig). To further investigate this, we performed a gene-level analysis (Methods). Interestingly, we found that most genes displayed consistent age-related effects across all osteoprogenitor types (S6C and S6D Fig). For instance, genes associated with bone formation, such as Bmp2, Chrdl1, and Itgb3, were upregulated with increasing age across all osteoprogenitors, while cell-cycle-related genes like Cdk8 and Parp1 were downregulated (S6C Fig). GSEA enrichment results indicated that pathways related to oxidative stress, hypoxia response, and lipid synthesis were upregulated across all osteoprogenitors with increasing age. Conversely, cell cycle activity, mRNA splicing, the Wnt pathway, and metabolism of glycine, serine, and threonine were downregulated (S6E Fig). These findings align with a previous study [36], indicating a conserved effect of age on osteoprogenitors, which is potentially linked to bone disorders like osteoporosis.
Differentiation Model provides a comprehensive understanding of osteogenic differentiation
To understand how various osteoprogenitor cells differentiate into osteoblasts differentially, we established a Differentiation Model that focuses on the genetic regulation and pathway dynamics during osteogenesis. In our Differentiation Atlas, a continuum of cellular states between osteoprogenitors and osteoblasts in both UMAP and force-directed graphs was observed, indicating that our atlas reconstructs cellular transitions during osteoblast differentiation (Figs 2A and 3A–3C). Besides, we observed that different samples shared similar transition processes from specific osteoprogenitors, supporting the grouping of these trajectories together for further analysis (S8A–S8C Fig). To distinguish these grouped trajectories from individual sample trajectories, we termed them OsteoProgenitor Cell-Specific Trajectories (OPCSTs). We first investigated which osteoprogenitor clusters could directly transition into osteoblasts. To achieve this, we employed coarse-grained connectivity structures to capture cell transitions [37] between osteoprogenitors and osteoblasts, with higher connectivity indicating a greater probability of transition. (Figs 3A and S8D). This approach identified four distinct grouped trajectories: Chondrocyte OPCST, LepR+ BMSC OPCST, Fibroblast OPCST, and Mes OPCST (Figs 3A and S8D).
Fig 3. Differentiation Model provides a comprehensive understanding of osteogenic differentiation.
(A) PAGA captures the cell transition of four osteoprogenitor clusters to osteoblasts through coarse-grained connectivity structures. (B) Force-directed graph of differentiation atlas colored by the endpoint of the four osteoprogenitor clusters. (C) Force-directed graph of differentiation atlas colored by differentiation path from the four osteoprogenitor cell-specific trajectories (OPCST). (D) Force-directed graph of differentiation atlas colored by common pseudotime of the four OPCST. (E) Heatmap showing the activity of six regulon clusters (row) across different pseudotime bins of four OPCST (column). (F) Differentiation Model reconstructs the differentiation process of osteoblast. Each node represents a pseudotime bin of OPCSTs, and colors represent regulon clusters in (E). pseudotime bins with similar scANVI representation are merged. (G-I), Activity of key pathway across four OPCSTs. The right panel depicts a trajectory dotplot of genes in the pathway, where the colors represent the correlation coefficient between gene expression and pseudotime; the size of the dots represents gene expression along the pseudotime; the shape indicates the pseudotime where maximum expression occurs. The left panel illustrates the pathway activity across four OPCSTs. The color represents pathway activity. Panel (F) was created with BioRender.com.
Next, to identify the endpoints of the developmental trajectories, we explore the earliest states of osteoprogenitors (Figs 3B and S9A–S9C and Note 3 in S1 Text). To determine a universal indicator of differentiation from various osteoprogenitors, we benchmarked multiple machine learning strategies and ultimately employed an LGBMR Regressor to build common pseudotime (Figs 3D and S10A–S10H and Note 4 in S1 Text). To build the final model, we divided the differentiation path based on osteoprogenitors and pseudotime into bins, then merged bins representing similar cell states (Figs 3F and S11B–S11H and Methods). Furthermore, we conducted gene regulatory network inference to predict potential transcription factors and infer the activity of key pathways involved in osteogenesis (Figs 3E–3L and S12A–S12L and Methods). Additionally, we created a trajectory dotplot to visualize the pseudotemporal expression patterns across multiple trajectories (Figs 3G–3L and S13A–S13I and Note 5 in S1 Text). The inferred trajectory path of the Differentiation Model shows significant overlap with the reported lineage tracing cells [7,28], and most of the inferred transcription factors (82/136) have been demonstrated to be associated with bone formation (S12M Fig and S5 Table). Besides, a large portion of differentiated genes (938/1852) have been annotated in bone databases [38–40], such as Phylobone (S14Q Fig and Methods and S6 Table), further validating the model’s ability to capture biologically relevant information about bone development.
Previous research has described the cellular transition between chondrocytes and osteoblasts [28]. This transition can be well illustrated in our model, represented as Chondrocyte OPCST (Fig 3A). In our model, the Chondrocyte OPCST primarily progressed through three stages: prehypertrophic chondrocytes, hypertrophic chondrocytes, and osteocytes [25,28] (Fig 3E). In this differentiation process, transcription factors and signaling pathways undergo significant changes. In terms of transcription factors, early differentiation is regulated by Sox9(+) and Sox8(+), which maintain the chondrocyte fate, while late differentiation is regulated by Tgif1(+) and Sox4(+), both of which are associated with parathyroid hormone [41,42] (Figs 3D and S12G and S12H). As for signaling pathways, PDGF signaling (Col9a1, Col6a3, Thbs3) is highly activated in the early differentiation process, while FGFR signaling (Fgfr3, Fgfrl1, Pik3r1) and VEGF signaling (Vegfa, Jup, Hspb1) are involved in the late differentiation process (Fig 3K and 3L). These results show a significant reprogramming in chondrocytes during the transition toward osteoblast differentiation.
In long bones, LepR+ BMSC cells are typically dormant but become osteogenesis-activated in response to injury [7]. Our model divides this process into three stages: LepR+ BMSC cells, pre-osteoblast cells, and osteoblasts (Figs 3A and S8D). Like Chondrocyte OPCST, we observed that transcription factors and pathways exhibited cascading changes. Foxc2(+) [43] and Stat5a(+) [44] regulate the early stage of differentiation (Figs 3D and S12E and S12F), while Sp7 and Runx2 regulate the late stage (Figs 3D and S12K and S12M). TGF-β signaling and growth hormone receptor signaling pathway were highly activated at early differentiation, while extranuclear estrogen signaling at late (Figs 3I and 3J and S14L). Apart from LepR+ BMSC, fibroblasts have attracted more and more attention in long-bone regeneration [35]. Our model delineated the Fibroblast OPCST, which illustrates this differentiation process in fibroblasts (Fig 3A–3D and S8D). Different from LepR+ BMSC, Pax1(+) [45] and Scx(+) [46] regulate the early differentiation, and the PDGF signaling pathway (Thbs4, Thbs2, Col6a6) was highly active during this stage (Figs 3D and 3G and S12A and S12B).
Mesenchymal cells are the main cellular source of both limb buds and heads in the embryo. Our model captured this process and depicted it as the Mes OPCST. At early differentiation, Twist1(+) [29] and Msx2(+) [32] were transcription factors that regulate this process, and the Hedgehog signaling pathway (Hhip, Tubb6, Tubb4b) exhibited high activity at this stage (Figs 3D and 3H and S12C and S15D). Interestingly, we found that mesenchymal cells differentiated to pre-osteoblasts sharing similar cellular states with pre-osteoblasts in LepR+ OPCST (S11E Fig). However, pre-osteoblasts in Mes OPCSTs demonstrated high activity in the Wnt signaling pathway (Wnt6, Wnt10a, Wnt2), whereas pre-osteoblasts in LepR+ BMSC OPCSTs exhibited high activity in the Complement Cascade (C3, C4b, Cfh) (S14N–S14P Fig). This difference suggested that different osteoprogenitors could retain part of their own identity for responding to the microenvironment even after transitioning into similar cell states.
Overall, our Differentiation Model reconstructs the osteoblast differentiation process from different osteoprogenitors and provides a systematic view of the diversity in gene regulatory networks and pathway activities during osteoblast differentiation.
TrajDiff detects covariate-related gene differences across multiple trajectories
It is now understood that throughout the process of differentiation, variations in both cell abundance and gene expression [21,47] are highly influenced by factors such as age [17] and tissue origin [48]. In multi-stage differentiation processes like osteoblast differentiation [3] (Fig 3F), it is pivotal to pinpoint the stage at which differential genes exert their influence. However, current methods [21,47] fail to infer the differential abundance and expression within specific differentiation stages, thus calling for the development of new tools. In the present study, we introduced TrajDiff, a novel tool specifically designed for performing differential pseudotime analysis across multiple samples, aimed at uncovering changes in cell abundance and expression throughout differentiation stages (Fig 4A). This algorithm enables us to detect age and tissue-specific variations in differentiation. Our benchmarking results demonstrated that TrajDiff could outperform existing algorithms, such as Lamian [22] and Condiments [47], in various aspects, including the detection of differential abundance (S15A–S15I Fig and Note 6 in S1 Text) and trend differences of gene expression (S16 and S17 Figs and Note 6 in S1 Text).
Fig 4. TrajDiff detects covariate-related gene differences across multiple trajectories.
(A) A overview for TrajDiff. (B) Study design for TrajDiff analysis. (C) Force-directed graph visualization illustrates the difference in cell abundance between the Young and Adult groups. (D), Heatmap illustrates the difference in cell abundance along pseudotime (column) in 37 samples (row) between two groups. The four rows of bottom annotation represent: the mean cell abundance of the two groups (row 1, row 2), differential abundance (row 3), and false discovery rate (FDR) (row 4). (E) Heatmaps are presented in four vertical panels to illustrate gene expression of the Young group (first panel) and Adult group (second panel), expression differences between two groups (third panel), and FDR (fourth panel). In each panel, rows represent genes, while columns represent pseudotime. Genes are categorized into 8 clusters based on their expression patterns. (F), Trajectory dotplot illustrates expression of GO-enriched genes from eight gene clusters in (D) across 37 trajectories. Panel (A) and (B) was created with BioRender.com.
Our previous analysis on osteoprogenitors indicated that the states of LepR+ BMSCs and mesenchymal cells could change with age (S6A–S6E Fig). Additionally, prior studies have shown significant remodeling of gene expression [49] and biological function [34, 49] in LepR+ BMSCs during adolescence. This evidence suggested that the differentiation process of LepR+ BMSC may vary across different ages. To explore this age-related heterogeneity, we applied TrajDiff to LepR+ BMSC OPCST. Samples younger than 3 months were defined as the “Young” group, while others were assigned to the “Adult” group, based on previous studies [34,49] (Fig 4B). Differential abundance analysis revealed higher cell density at the late stage (preosteoblast and osteoblast) in the Adult group compared to the Young group, where cell density was higher at the early stage (LepR+ BMSC) (Fig 4C and 4D). This observation was unlikely to be due to technical sampling bias, as it has been consistently observed across multiple projects (Fig 4D). These results suggested that LepR+ BMSC cell abundance was low during postnatal and adolescent stages, which might explain why LepR+ BMSCs are not the main source of osteoblasts in the long bones of young mice during adolescence [34].
Using TrajDiff, we next performed differential expression analysis to identify genes whose expression varied across differentiation stages. Our study identified 74.2% of the differentially expressed genes reported in the previous study [49] (S18J Fig). While the previous study provided a binary classification of upregulated or downregulated genes [49], our results offer a more comprehensive understanding by elucidating the precise developmental stages at which these genes exhibit differential expression patterns. We categorized these differentially expressed genes into eight groups based on their expression patterns (S18B–S18J Fig). We noticed that some genes are differentially expressed during the whole differentiation process. For example, genes related to leukocyte cell−cell adhesion, such as Smad7, Hspb1, and Pla2g5, showed higher expression in the Adult group than the Young group during differentiation (“Down_Down”) (Figs 4D and 4E and S18A and S18G). While some genes are only differentially expressed at the specific differentiation process. Genes involved in ameboidal−type cell migration (P2rx4, Mmp12, Slit2), for instance, were downregulated only at the early stage (“Down_0”) (Figs 4D and 4E, S18A and S18F). Interestingly, we noticed that certain genes were highly expressed in one group during early differentiation, but then became highly expressed in the other group during late differentiation. Genes related to TGF-β production (Itgb8, Cx3cl1, Fbln1) and hormone activity (Agt, Bglap, Bglap2) are both of this type, which suggests the roles of signaling pathways vary depending on age (Figs 4D and 4E and S18A, S18E, S18H and S18I). These findings corroborate previous research that reported enrichment of pathways associated with hematopoiesis, inflammatory responses [49], and antigen processing in older mice, whereas our results provide higher resolution insights across developmental stages.
Next, we investigated genes with transient differential expression. These genes, representing a smaller proportion (357 genes) compared to persistently differentially expressed genes (876 genes), exhibited dynamic changes throughout differentiation (S17A and S17C Fig). In the Young group, transiently upregulated genes at the early stage were associated with neuronal activity (Clstn2, Flrt3, Shisa9) (S17A and S17B Fig). Conversely, genes transiently downregulated at the early stage were associated with morphogenesis (Sox9, Lhx1, Spg11) (S17A and S17B Fig). Additionally, Hes1, involved in cell differentiation, was downregulated at the middle stage, while Stk17b, associated with fibroblast apoptosis, was downregulated at the late stage (S17A and S17B Fig).
In summary, TrajDiff offers a distinct advantage over Lamian and Condiments by identifying differential genes at precise developmental stages. Consequently, it furnishes a comprehensive landscape of age-related gene expression dynamics during the differentiation of LepR+ BMSCs.
TRAVMap reveals pseudotemporal gene module heterogeneity across all trajectories
During differentiation, groups of genes that participate collectively to execute specific functions are referred to as gene modules [50]. However, current gene module detection methods can’t integrate multiple datasets to yield more reliable results. To address this limitation, we developed TRAVMap, a method for identifying differentiation-related gene modules across multiple trajectories (Fig 5A). TRAVMap utilizes pseudotime as an axis, and by projecting gene expression onto this axis, it identifies recurring axes of variation [51] within these trajectories, termed Trajectory-related Replicable Axes of Variation (TRAVs) (Fig 5A). We found that genes that drive TRAVs show coordinated expression patterns, representing gene modules that function at specific stages of the differentiation process (S20D, S20F and S20H Fig). Additionally, these gene modules are conserved across multiple samples, demonstrating the robustness and effectiveness of our algorithm (S20H Fig). TRAVMap distinguishes itself from existing gene module detection algorithms in two key ways: (1) it integrates multiple samples to derive more robust gene modules, and (2) it focuses specifically on pseudotemporal dynamics, offering insights into gene function across the temporal progression of differentiation. In this way, TRAVMap identified 226 TRAVs across 121 trajectories (Fig 5B). Furthermore, by leveraging TRAVs and gene expression data, TRAVMap learns representations of trajectories to capture sample-to-sample variability, allowing us to identify the sources of variability. Unlike current deep learning approaches [52], our approach incorporates gene and gene module activities, making it interpretable and providing a detailed view of gene expression dynamics and gene module activity across population-level trajectories (S19A–S19E Fig and Note 7 in S1 Text). Our methods facilitate multi-scale exploration of genes and gene modules in differentiation processes.
Fig 5. TRAVMap reveals gene module heterogeneity across all trajectories.
(A,B) An overview for TRAVMap. (B-E) Trajectory embeddings visualized with UMAP, colored by OPCST, Age, Tissue origin, and Tissue location. Each point represents a trajectory. (G,J) GO enrichment of Trajectory related Replicable Axis (TRAV) gene module, visualized with dotplot. The bottom dot annotation represents the differentiation stage at which the gene module executes its function. (H,K) TRAV activity with UMAP visualization in (B-E) (I,L) Trajectory dotplot illustrates the gene expression patterns across 20 randomly sampled trajectories from four OPCST.
TRAVMap reveals that osteoprogenitor cell identity, age, and tissue origin can all drive heterogeneity in osteoblast differentiation processes (Figs 5C–5F and S19A–S19E). Among these factors, the identity of the osteoprogenitor cells contributes the most to this observed heterogeneity. Therefore, we identified dozens of OPCST-associated gene modules. We focused on gene modules in LepR+ BMSC OPCST and found that these gene modules function at specific stages of the differentiation process, representing distinct biological processes. For example, TRAV122 functions at the early differentiation process, enriched genes associated with taxis; while TRAV143 functions at the middle stage of differentiation, enriched genes involved in the structure of the extracellular matrix (Fig 5G and 5H). To examine the expression pattern of the TRAV143 gene module, we randomly selected five trajectories for each OPCST and visualized them using a trajectory dotplot (Fig 5I). We observed that most of the genes within this module exhibited high expression that peaked at the middle stage of differentiation, specifically in the LepR+ BMSC OPCST trajectories, whereas they exhibited distinct pseudotemporal expression patterns in trajectories derived from other OPCST (Figs 5I and S20H). This confirms that the TRAV143 gene module functioned during the middle stage of differentiation, specifically in the LepR+ BMSC OPCST (Figs 5H and 5I and S20D).
In this way, we identified osteogenesis-conserved gene modules and age-related gene modules. The osteogenesis-conserved gene modules were active across most of the osteoblast differentiation trajectories, representing the conserved genetic programs driving the osteogenic process (Figs 5J and S20A). We found that most of these gene modules functioned at the late stage of differentiation, including TRAV208, TRAV77, and TRAV6, which were associated with biological processes like ossification, bone mineralization, and growth factor binding (Fig 5J). The age-related gene modules differentially activate between age groups. One example was TRAV140, which exhibited differential activity across LepR+ BMSC, Fibroblast, and Mes OPCSTs (S21A and S21B Fig). In postnatal and young adult LepR+ OPCST trajectories, TRAV140 showed high activity, and its associated gene module, containing genes like Smpd3 and Ano6, reached peak expression at the late stage (S21D and S21F Fig). However, in adult and old groups, TRAV140 activity was lower, and the gene module exhibited inconsistent expression patterns (S21D and S21G Fig). Notably, a majority of genes (61 out of 100) within the TRAV140 module overlapped with genes identified by TrajDiff, further supporting its age-related nature (S21E Fig). Gene Ontology analysis suggested that this module was associated with bone morphogenesis and stem cell development, implying potential differences in osteogenesis activity across age groups (S21H Fig). Interestingly, the predicted transcription factors regulating TRAV140, Fosl1(+) [53], and Lef1(+) [54], represented promising therapeutic targets for age-related bone disorders like osteoporosis (S21I Fig).
In conclusion, TRAVMap effectively identified both osteoprogenitor-related and age-related gene modules. This allowed for the creation of a landscape of pseudotemporal gene modules that are active at distinct developmental stages across population-level differentiation trajectories.
Applying TrajAtlas to an extended atlas elucidates alterations in trajectories under injury conditions
The Differentiation Atlas is primarily designed to understand the osteogenesis process in healthy mice, aiming to establish a reference model for normal osteoblast differentiation. However, osteoblast differentiation can be altered or disrupted by various conditions, including injury [7,55], and disease [55]. Projecting new datasets onto a reference atlas has been shown to effectively detect aberrant cell states [56]. To investigate osteogenesis within diverse microenvironments, we collected public datasets that incorporated a broader range of tissues (e.g., digit bone, rib, periodontium), encompassed more complex cellular states (such as injury, heterotopic ossification, and disease), and included data from multiple species (human and rat) (S22A–S22F Fig and S1 Table). Then, we mapped our extended datasets to the Differentiation Atlas using scArches, a reference mapping approach (Fig 6A). The final integrated datasets comprises 319 samples and 781,397 cells, which we refer to as the extended atlas.
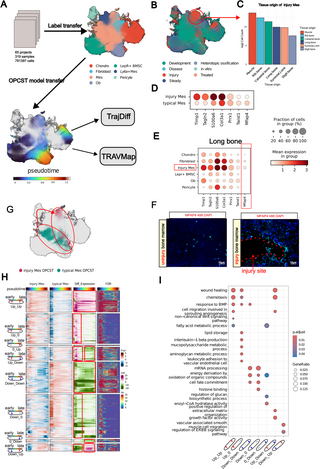
Fig 6. Applying TrajAtlas to an extended atlas elucidates alterations in trajectories under injury conditions.
(A) An overview of extended atlas construction. (B) UMAP visualization of the extended atlas, colored by stage, highlighting cells (Injury Mes) that group together in the injury state. (C) Barplot shows the cell count (log2 scale) of tissue origin for Injury Mes in (B). (D) Dotplot displays the expression levels of Mes marker genes and differentially expressed genes between typical Mes and injury Mes. (E) Dotplot shows expression levels of marker genes of injury Mes across all cell types present in long bone datasets. (F) MFAP4 (green) in 8-week-old femur from injured and uninjured conditions. (G) UMAP visualization of Mes OPCST between injury and typical states. (H) Heatmaps are presented in four vertical panels to illustrate gene expression of the injury Mes group (first panel) and typical Mes group (second panel), expression differences between the two groups (third panel), and FDR (fourth panel). (I) GO enrichment of seven clusters in (F), visualized with dotplot.
Following data integration and label transfer, most cells were confidently annotated with level-2 labels (Figs 6A and S23E). However, we observed high uncertainty associated with a specific mesenchymal stem cell state, primarily composed of cells from injury datasets (Figs 6B and S23A–S23F). Notably, this state, termed “injury Mes”, appeared to originate from various injured tissues such as skeletal muscle, rib bone, and calvarial bone (Figs 6C and S23G). This suggests that cells from different tissues may be reprogrammed to a Mesenchyme-like state upon injury, reminiscent of blastema formation in salamanders [57]. In salamanders, cells can dedifferentiate and acquire pluripotency to regenerate tissues. Interestingly, in this study, injured Mes expressed typical Mes marker genes, such as Prrx1 [29] and Twist1 [29] (S23H and S23I Fig), but also exhibited high expression of genes related to angiogenesis, such as Tagln2 [58], which distinguished them from typical Mes (Figs 6D and S23J). Among the tissues that give rise to injury Mes, we validated these results specifically on the long bone. We chose Mfap4 as a marker gene, as it is specifically expressed in injury Mes in the long bone (Fig 6E). Immunohistochemistry results showed that Mfap4 is highly expressed in the long bone after injury, and is localized near the injury sites (Fig 6F). In contrast, Mfap4 expression was rarely detected in uninjured long bones (Fig 6F).
Building on previous research that identified osteogenic potential in cells from injured tissues like skeletal muscle [59] and calvarial bone [60], we applied the Differentiation Model to the extended atlas (Fig 6A). This analysis revealed a distinct trajectory for injury-derived Mes OPCSTs transitioning towards osteoblasts, deviating from the trajectory of typical Mes OPCSTs (Fig 6G). We employed TrajDiff to explore the heterogeneity in the differentiation processes between these two populations. The cell abundance analysis suggested that injury-derived Mes OPCSTs exhibited higher density in the early stages of differentiation, but this density was lower in the late stages (S24A Fig). This pattern was particularly evident in tissues like skeletal muscle, where cells in the late stages were scarce (S24A Fig). These findings suggest that injury-derived Mes might have a slower differentiation speed [47] than typical Mes.
Next, we performed a differential expression analysis using TrajDiff, which revealed distinct gene expression patterns. Genes associated with wound healing (e.g., Mia3, Hif1a, Smoc2) and chemotaxis (e.g., Cxcl9, Lrp1, Ecscr) were consistently upregulated throughout differentiation in injury-derived Mes (Up–Up) (Figs 6H and 6I and S24B). Conversely, genes involved in cell fate commitment (e.g., Hes1, Bcl11b, Id2,) were downregulated (Down-Down) (Figs 6H and 6I and S24B). This suggests that injury may steer these cells away from terminal differentiation states. Additionally, genes related to interleukin-1 beta production (e.g., Cd36, Ccl3, Igf1) showed early upregulation (Up_0), indicating a potential role in the initial response to injury (Figs 6H and 6I and S24B). Interestingly, genes involved in growth factor activity (e.g., Ogn, Hbegf, Clec11a) displayed a transient upregulation pattern, being upregulated early but downregulated later, suggesting a stage-specific role for growth factor activity in injury Mes differentiation (Figs 6H and 6I and S24B).
We then applied TRAVMap to our extended atlas (S25A–S25F Fig). This analysis identified three injury-related gene modules (S25G–S25K Fig). These modules exhibited high activity specifically in injury trajectories, primarily functioning at the early stage of differentiation (S25L Fig). GO enrichment analysis revealed that these modules (TRAV540, TRAV766, TRAV767) are associated with distinct processes: immune response (Sema7a, Rsad2, Tek), bone development (Scx, Acp5, Fam20c), and cell proliferation (Klf4, Egr1, Hpgd), respectively (S25M Fig).
Discussion
In this study, we introduce TrajAtlas, a novel framework centered on trajectories to analyze differentiation heterogeneity. Unlike previous methods, TrajAtlas prioritizes trajectories to build a comprehensive landscape of osteoblast differentiation, representing the first framework to systematically dissect large-scale trajectory heterogeneity. TrajAtlas allows multi-scale exploration of differentiation, examining both individual cells and entire samples. This deepens our understanding of stem cell heterogeneity and how trajectories influence cell density, gene expression, and gene modules. Our key technical innovations include: (1) integrating large-scale trajectories to construct a reference atlas and a universal differentiation model, (2) developing statistical tools to identify differentially expressed genes along trajectories, and (3) implementing methods to infer conserved pseudotemporal gene modules across population-level samples. Additionally, we established methods to visualize changes in gene expression and gene modules across population-level datasets. To guarantee the framework’s universality for osteogenic processes, we initially built a core reference atlas using data from three tissues. We then successfully validated the framework on the extended atlas with various osteogenic datasets.
The major challenge in skeletal biology lies in the diversity of osteoprogenitor cells [5,6]. Due to a lack of comprehensive annotation, the characterization of responding trajectories has been a subject of controversy for a long time [5–7,25,34]. Our work addressed this gap by mapping experimentally validated osteoprogenitors onto our detailed differentiation atlas, which utilizes a seven-level annotation system. This analysis categorized osteoprogenitors into six major clusters, with at least four exhibiting the potential to directly transform into osteoblasts. We further identified key transcription factor groups that differentially regulate this transformation process, termed osteoprogenitor cell-specific differentiation trajectories. By investigating several crucial pathways for osteoblast differentiation, we revealed distinct activity patterns during osteogenesis, influenced by both the cellular source of the osteoprogenitors and the stage of differentiation. For example, the Hedgehog signaling pathway, known for its role in bone formation and maintenance [61], displayed varying activity levels during the early stages of Mes OPCSTs, with Hhip and Tubb6 identified as potential target genes. This OPCST-dependent pathway activity suggested an osteoprogenitor-specific approach for selecting therapeutic targets for bone diseases [62]. Finally, by applying TRAVMap, we identified dozens of pseudotemporal gene modules that function sequentially throughout OPCST differentiation. This work sheds light on the dynamic interplay between genes and gene modules as differentiation progresses.
Bone formation and bone disease are closely related to age [17,34] and tissue location [5,6]. In this study, we investigated the influence of age and tissue location on bone formation and disease, focusing on four key aspects: cell type composition, osteoprogenitor state, differentially expressed genes along osteoprogenitors’ differentiation trajectories, and gene modules. We identified universal age-related hallmarks across osteoprogenitors, such as cell cycle activity and Wnt pathway activation, which aligned with previous findings on senescence [36]. TRAVMap analysis revealed a similar conserved effect in gene modules, consistently associating specific modules with age across multiple OPCSTs. This age-related influence was likely mediated by Fosl1(+), suggesting potential therapeutic targets for age-related bone diseases like osteoporosis. Notably, TrajDiff analysis of LepR+ BMSC OPCSTs revealed differentially expressed genes categorized by their expression patterns throughout differentiation. This finding highlights the highly specific and variable effect of age on different OPCSTs and differentiation stages.
Several tissues are widely thought to contribute to bone regeneration, including bone marrow [7], periosteum [35], and skeletal muscle [59]. By analyzing datasets from injured tissues, we identified an injury-related Mesenchyme-like cellular state. This phenomenon resembled blastema formation observed in salamanders [57] and fish [63], where blastemas arise from the dermis, cartilage, and muscle cells. Interestingly, mature osteoblasts were only observed in datasets derived from long bones and calvarial bones, suggesting a potential loss of regenerative capacity in mammals [64]. Compared to typical Mes, injury Mes exhibited altered differentiation trajectories. Using TrajDiff, we identified genes and gene modules associated with injury, providing valuable insights into bone regeneration processes.
While our framework is primarily designed to analyze osteoblast differentiation, it has the potential to be adapted for studying other differentiation processes, cell reprogramming, and even the cell cycle. Traditional “cell-centric analysis” offers a snapshot of cell type and gene expression at a single point in time. In contrast, “trajectory-centric analysis” sheds light on dynamic processes by examining changes over time. In summary, our study introduces a novel trajectory-centric framework that provides new insights into the dynamic interplay between cells, genes, and gene modules during osteogenesis.
While TrajAtlas offers a robust framework for modeling differentiation across large-scale datasets, there are still limitations to address. First, although our reference atlas includes datasets from a variety of tissues, it lacks finer spatial resolution. This finer resolution is crucial for understanding differentiation within specific niches and for elucidating processes such as cell communication [20] and angiogenesis [65] that are related to differentiation. However, with the advancements in spatial omics, constructing a more detailed spatial atlas [66] soon seems promising. Second, our atlas primarily focuses on the transition of different cell states into osteoblasts. However, this transition is likely not unidirectional [5]. For example, bone marrow stromal cells (BMSCs) can differentiate into chondrocytes [25], and chondrocytes can also differentiate to BMSCs [67]. The multidirectional transitions among various osteoprogenitors are also critical aspects of osteogenesis. Given that single-cell datasets alone make it challenging to recover these multidirectional cellular transitions, incorporating sequencing-based lineage-tracing methods [68] holds promise for developing more complex models that can reveal intricate differentiation dynamics soon.
Supporting information
S2 Fig. Selection of single-cell integration method and scANVI hyperparameters.
A, Result of data integration benchmarking. The rows represent methods tested, using a particular preprocessing and output. Preprocessing is summarized by “Scaling” (specifying whether or not gene values were scaled to mean 0 and standard deviation 1 across cells). Methods are sorted by overall score. The overall score is a weighted mean of the batch correction score and the bio-conservation score, which in turn are a mean of the individual metrics within the category. The output column specifies whether a method has corrected gene counts, an integrated embedding, or an integrated graph as output. B, Result of hyperparameters (HVG, n_latent) of scANVI benchmarking. The rows represent hyperparameters tested. C,D, Cell clusters annotated with previous studies are well separated with UMAP reduction in scANVI latent space.
https://doi.org/10.1371/journal.pgen.1011319.s002
(TIF)
S3 Fig. A full seven-level annotation system of the Differential Atlas. A, A full seven-level hierarchical tree of clusters of Differential Atlas.
The first five levels with up to 49 clusters are presented, emphasizing the diverse nature of OPCs across various tissues and age groups. The left heatmap (red) depicts the overlapping of experimentally validated OPCs with clusters in the lowest tree level in the Differentiation Atlas. The middle heatmap (orange) depicts the relative percentage contribution of each cluster at the lowest tree level to the age group. The right heatmap (dark green) illustrates the relative percentage contribution of each cluster to the tissue origin group.
https://doi.org/10.1371/journal.pgen.1011319.s003
(TIF)
S4 Fig. Seven-level annotation system harmonized annotations from previous studies and different tissues.
A, Sankey plot shows how level-2 annotations harmonize cell types from different tissues (LA: long bone; LE: Limb bud, C: Head). B-H, Barplot shows that level-2 annotations and level-3 annotations harmonize cell types from different studies.
https://doi.org/10.1371/journal.pgen.1011319.s004
(TIF)
S5 Fig. Examples of mapping experimentally validated OPCs to Differential Atlas.
A-E, Examples of mapping experimentally validated OPCs to Differential Atlas. Left panels show the information on experimentally validated OPCs. The middle panels illustrate the cells mapped based on marker expression and tissue locations with UMAP visualization. The left panels show the expression of OPCs’ markers with UMAP visualization.
https://doi.org/10.1371/journal.pgen.1011319.s005
(TIF)
S6 Fig. Age-related variation in OPCs states.
A, Barplots show the cell proportion of Mes subpopulations across different age groups. B, Fraction of total inter-sample variance in the Differential Atlas embedding that correlates with specific covariates. Covariates are split into technical (left) and biological covariates (right). Cell types at second annotation levels are shown. C, Heatmap shows differential expression z-statistic for genes in each cell cluster. ‘*’ indicates study-wide FDR < 5% in all panels. Grey boxs indicates a gene did not pass the expression cutoff in that cell cluster. Genes are categorized based on whether they are concordantly different across five OPCs. D, Barplot shows the number of genes in three categories in (C). E, Gene set analysis using the full spectrum of test statistics shows cell type conserved signatures Study-wide FDR < 0.05 is indicated by ‘*’.
https://doi.org/10.1371/journal.pgen.1011319.s006
(TIF)
S7 Fig. Differentiation path reconstruction in Differentiation Model.
A,F,K,N, Force-directed graph visualization of differentiation path of (A) Chondrocyte OPCST, (F) LepR+ BMSC OPCST, (K) Fibroblast OPCST and (N) Mes OPCST. B,G, Force-directed graph highlighted lineage-tracked cells of (B) Col10a1, (G) Cxcl12, C-E,H,J,L-M,O-P Force-directed graph visualization of gene expression that is high along the differential path.
https://doi.org/10.1371/journal.pgen.1011319.s007
(TIF)
S8 Fig. Cell transitions between OPCs and osteoblasts were captured by PAGA connectivity structures.
A-C, Transition processes from specific osteoprogenitors are similar across samples.D, Heatmap shows PAGA connectivity between four OPCs (column) and osteoblasts in samples (row).
https://doi.org/10.1371/journal.pgen.1011319.s008
(TIF)
S9 Fig. Endpoint identification of Differentiation Model.
A, Expression of adult stem cell markers visualized with force-directed graph B, Barplot showing cell proportion in different ages across level-5 annotation C, Vlnplot shows the developmental potential predicted by CytoTRACE. Lower values indicate higher potential.
https://doi.org/10.1371/journal.pgen.1011319.s009
(TIF)
S10 Fig. Common pseudotime predicted by the LGBMR Regressor can effectively reconstruct osteoblast differentiation progression.
A, Expression of adult stem cell markers visualized with force-directed graph B, Barplot showing cell proportion in different ages across level-5 annotation C, Vlnplot shows the developmental potential predicted by CytoTRACE. Lower values indicate higher potential.
https://doi.org/10.1371/journal.pgen.1011319.s010
(TIF)
S11 Fig. Construction of Differentiation Model.
A,Diagram of the three hypotheses. B-D, Force-directed graph visualization colored by (B) 10 pseudotime bins, (C) pseudotime bin 4 in OPCSTs, and (D) pseudotime bin 5 in OPCSTs. E-G, Pseudotime bins across different OPCSTs with similar states are merged. H.,Final Differentiation Model inferred by (E-G). This figure was created with BioRender.com.
https://doi.org/10.1371/journal.pgen.1011319.s011
(TIF)
S12 Fig. Differentiation model reveals that transcription factor activities vary across different OPCSTs.
A-L, The left panel illustrates the activity of transcription factors across four OPCSTs. The colors represent pathway activity. The transcription factors to show were selected from Fig 3E. M, Barplot shows that most transcription factors identified in the Differentiation Model were validated to be related to bone formation (S5 Table). This figure was created with BioRender.com.
https://doi.org/10.1371/journal.pgen.1011319.s012
(TIF)
S13 Fig. The trajectory dotplot enables visualization of gene expression across multiple trajectories while retaining most information.
A, Sixteen attributes were extracted from gene expression along the pseudotime. B, The barplot shows the random forest-predicted importance of attributes in reconstructing gene expression. C, Scatter plot demonstrates that selected attributes can reconstruct GAM-fitted expression (left) and raw count (right). D-I, Trajectory dotplot intuitively reflects the different pseudotemporal expression patterns of genes. Pseudotemporal gene expression was visualized with CellRank (left panel) and trajectory dotplot (right panel).
https://doi.org/10.1371/journal.pgen.1011319.s013
(TIF)
S14 Fig. Differentiation model identified osteoprogenitor cell-specific pathways.
A, Multiway heatmap of changes of pseudotemporal gene expression for four OPCST. Genes to show were selected with the associationTest procedure from tradeSeq. Gene clusters were grouped by k-means. B-M, The barplots shows the top five Reactome enrichment results of gene clusters in (a) arranged by p-value. N, Pre-osteoblasts between Lepr+ BMSC OPCST and Mes OPCST exhibit different states. O,P, Barplots show the top five Reactome enrichment results of differential genes between two states of pre-osteoblast in (N) arranged by p-value Q, Venn plot shows the major proportion of differential genes identified in Differentiation Models are annotated in other bone databases (S6 Table).
https://doi.org/10.1371/journal.pgen.1011319.s014
(TIF)
S15 Fig. Benchmarking results of differential cell abundance analysis.
A-C, Overview of benchmarking strategy for (A) accuracy, (B) specificity, and (C) local variation detection. D, Heatmap reveals that TrajDiff can identify subtle changes in cell abundance. E, Heatmap reveals that TrajDiff exhibits robustness to false positives. F, Heatmap reveals that TrajDiff identifies local variations in cell abundance. G-I, TrajDiff demonstrates both high accuracy and specificity compared to Lamian and Condiment.
https://doi.org/10.1371/journal.pgen.1011319.s015
(TIF)
S16 Fig. Benchmarking results of differential expression analysis.
A-C, Overview of benchmarking strategy for (A) mean difference, (B) trend difference, and (C) generality. B, TrajDiff performs better in detecting mean difference and trend difference than Lamian. C, TrajDiff takes significantly less time compared to Lamian.
https://doi.org/10.1371/journal.pgen.1011319.s016
(TIF)
S17 Fig. TrajDiff detects genes that exhibit transient differential expression.
A, Heatmaps are presented in four vertical panels to illustrate transit differential genes expression of the Young group (first panel) and the Adult group (second panel), expression differences between the two groups (third panel), and FDR (fourth panel). In each panel, rows represent genes, while columns represent pseudotime. Genes are categorized into 8 clusters based on their expression patterns. B, GO enrichment of six gene clusters in (A), visualized with dotplot. C, The number of genes in different differential patterns (persistent or transitional).
https://doi.org/10.1371/journal.pgen.1011319.s017
(TIF)
S18 Fig. TrajDiff detects differential genes present across different differentiation stages.
A, GO enrichment of seven clusters in Fig 4D, visualized with dotplot. B-I, Pseudotemporal gene expression visualized with CellRank to validate differential genes in Fig 4E. J, Venn plot shows the overlap between differential genes in TrajDiff and differential genes provided in the previous study.
https://doi.org/10.1371/journal.pgen.1011319.s018
(TIF)
S19 Fig. Trajectory reduction enables visualization of gene expression and TRAV activity on population-level trajectories.
A, A schematic for trajectory reduction. TRAV activity matrix, gene expression, gene peak, and gene correlation were treated as four modalities of trajectories, which were integrated by weighted nearest neighbor (WNN) to get trajectory embeddings. B-E, Independent analysis of (B) peak, (C) expression, (D) correlation, and (E) TRAV modalities. F-I, Trajectory reduction after integrating four modalities with WNN, colored by (F) Tissue location, (G) Machine, (H) Project, (I) Treatment. J-M, Trajectory reduction enables visualization of (J) TRAV activities, (K) gene expression, (L) gene correlation, and (M) gene peak.
https://doi.org/10.1371/journal.pgen.1011319.s019
(TIF)
S20 Fig. TRAVMap identifed OPCST-related TRAVs.
a, Heatmap shows activity of TRAVs (row) across trajectories (column) of four OPCST. b,c, TRAV activity visualized with trajectory embeddings, colored by (b) LepR+ BMSC OPCST TRAVs, (c) osteogenesis-conserved TRAVs. d,f, Heatmap shows pseudotemporal gene expression of (d) TRAV143 and (f) TRAV77 in the sample with the highest TRAV activity. e,f, Predicted transcription factors that regulate (e) TRAV143, (g) TRAV77. h, Heatmaps shows shows conserved pseudotemporal gene expression of TRAV143 in multiple samples.
https://doi.org/10.1371/journal.pgen.1011319.s020
(TIF)
S21 Fig. TRAVMap identfied age-related TRAVs.
A, Heatmap shows the activity of TRAV140 across OPCSTs (row) and Ages (column). B, Violin plots show TRAV activity across OPCSTs and Ages (row). C, TRAV140 activity visualized with trajectory embeddings. D, Trajectory dotplot illustrates the genes expression patterns of the TRAV140 gene module (row) across LepR+ OPCST trajectories (column). E, Venn plot shows overlapping between genes in TRAV140 gene module and genes identified with TrajDiff in Fig 4D. F,G, Heatmap shows distinct pseudotemporal gene expression of TRAV140 gene module in trajectories in (F) postnatal group and (G) adult group. H, GO enrichment of TRAV140 gene module, visualized with dotplot. i, Predicted Transcript factors that regulate TRAV140.
https://doi.org/10.1371/journal.pgen.1011319.s021
(TIF)
S23 Fig. A Mes-like cellular state that related to injury was identified in extended atlas.
a, UMAP visualization of jointly embedded Differential Atlas (core) and the projected datasets (Extend) b, UMAP visualization of level-2 annotation in Differential Atlas. c, UMAP visualization of uncertainty score (Methods) d, UMAP visualization to highlight typical Mes and injury Mes. g, Heatmap visualization of cell count (fist columns) and metadata of injury Mes in (d). f,h, UMAP visualization of typical Mes marker. i, GO enrichment of differential genes between injury Mes and typical Mes, visualized with dotplot.
https://doi.org/10.1371/journal.pgen.1011319.s023
(TIF)
S24 Fig. Identification of differential genes in Mes OPCST between injury and typical States using TrajDiff on extended atlas.
A, Heatmap illustrates difference in cell abundance along pseudotime (column) in Mes OPCST trajectories (row) between injury and typical state. The four rows of bottom annotation represent: Mean cell abundance of the two groups (row 1, row 2), Differential abundance (row 3), and False discovery rate (FDR) (row 4). B, Trajectory dotplot illustrates expression of GO-enriched genes from seven gene cluster in Fig 6F across Mes OPCST trajectories.
https://doi.org/10.1371/journal.pgen.1011319.s024
(TIF)
S25 Fig. Identification of injury-related TRAVs using TRAVMap on extended atlas.
a-f,h, Trajectory embeddings visualized with UMAP, colored by (a) OPCST, (b) Age, (c) deriving from the Differentiation Atlas or projected datasets, (d) Machine group, (e) Species (f) Tissue origin, and (h) State. g, Volcano plot shows injury-related TRAVs. i-f,TRAV activity visualized with trajectory embeddings, colored by injury-related TRAV. l, Trajectory dotplot illustrates expression of genes of TRAV 767 gene module across Mes OPCST. mGO enrichment of three TRAV gene modules, visualized with dotplot.
https://doi.org/10.1371/journal.pgen.1011319.s025
(TIF)




Add Comment