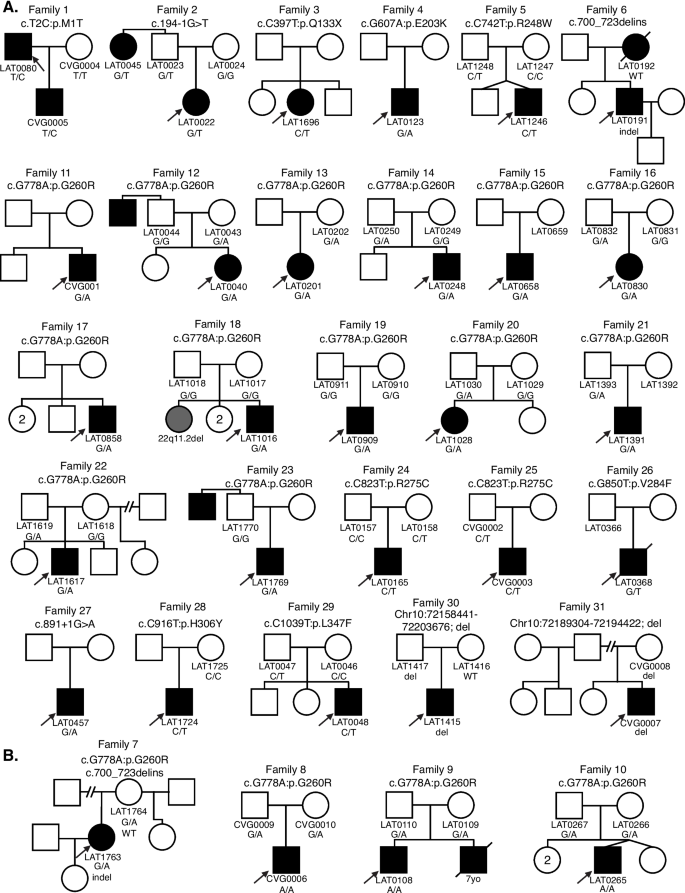
Genotypic and phenotypic expansion of NODAL SNVs/INDEL in laterality defects
The majority of cases were collected from a prospective study of congenital cardiac laterality defects. Proband exome sequencing was available in 321 of these subjects. While a NODAL SNV or indel had been already reported in 10 of these subjects by our group [13, 15], re-analysis detected an additional 11 cases, for a total of 21/321 (6.5%) cases with a NODAL variant and laterality CHD. An additional 6 cases with a NODAL variant were included that were previously reported by our group using single-gene sequencing for NODAL of probands from a separate subcohort of laterality CHD [13]. For these 27 probands from Group 1 and the 4 probands from Group 2 who had incomplete familial testing, we performed Sanger dideoxy sequencing for variant allele confirmation and segregation analysis in available family members. The NODAL variant was inherited in 19 (including 3 homozygous and 1 compound heterozygous inheritance), de novo in 2, and inheritance data unknown in 10 probands because of lacking information from one or both parents.
Analysis of relatives also revealed an unreported case of an affected aunt (LAT0045, Family 2) with laterality CHD and a NODAL variant (Fig. 1). In Group 2, one infant subject that underwent clinical testing (CVG0005) was a son of a proband (LAT0080) in the larger 321-person (Group 1a). These combined cohorts and parental evaluation resulted in 31 unrelated families with NODAL variants; 33 subjects with CHD (Additional file 1: Tables S1 and S2).
Comprehensive pedigrees and their genotypes for families with NODAL variants. Standard pedigree structures are utilized—filled circles and squares denote clinically affected individuals, and probands are indicated by black arrows. A Pedigrees of probands harboring heterozygous NODAL variants. B Pedigrees of probands harboring biallelic NODAL variants
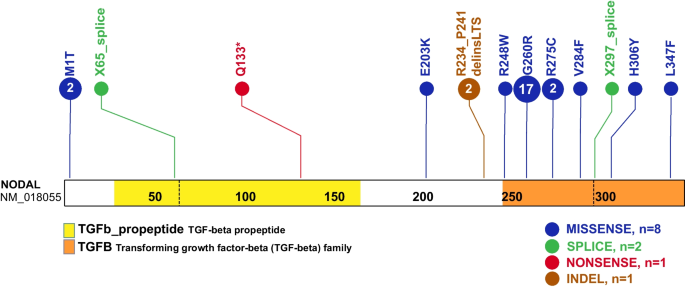
A total of eleven NODAL SNVs (8 missense, 2 splice site, and 1 nonsense) were detected in 31 laterality CHD cases from 29 families (Table 1, Fig. 1). These variant alleles mainly were localized to the portions of the protein constituting the TGF-β mature domain (Fig. 2). Cases with NODAL variants showed various CHD lesions, all of which had abnormal ventricular looping and/or abnormal great artery relationship (Table 2—summary, Additional file 1: Table S3-detailed). Within the laterality cohort, NODAL variants were most frequently observed in cases with congenitally corrected transposition of the great arteries (CCTGA), also known as levo-transposition of the great arteries (LTGA) (15.4%) and least frequently in cases with simple dextro-transposition of the great arteries (DTGA) (6.1%) and double outlet right ventricle with malposition of the great arteries (0.0%) (Table 3).
Schematic diagram of NODAL protein structure from conceptual translation of transcript NM_018055 with mapping location (vertical lollipops) of the exact map position of amino acid variants observed in this study. In total, we identified twelve different SNV alleles and indel that are distributed among the NODAL domains. The yellow horizontal rectangle represents the TGF-beta propeptide (amino acid 29-166) and the orange horizontal rectangle represents the mature transforming growth TGF-beta domain (amino acid 247-347). Blue circles denote missense variants, green circles denote splice site variants, red circles denote nonsense variants, and brown circles denote a complex indel variant. Numbers inside the circles refer to the variant frequency in our cohort
Two of the identified NODAL missense variants (c.2T>C, p.M1T; c.1039C>T, p.L347F) represented unreported variant alleles that have not been previously associated with laterality defects. The heterozygous p.M1T, identified in proband (LAT0080), was transmitted to his affected son (CVG005). This variant was associated with intrafamilial variable phenotypic expressivity in the proband and his son. The proband LAT0080 presented with heterotaxy, asplenia, right atrial isomerism, mitral atresia, ventricular septal defect (VSD), DORV with D-malposed great arteries (D-MGA), and pulmonary stenosis (PS). He also developed severe arteriovenous malformations (AVMs) and vesicoureteral reflux (VUR); the latter for which he underwent post ureteral reimplantation surgery. Whereas his son (CVG005) exhibited CCTGA with L-ventricular looping, LTGA, pulmonary atresia, and VSD (Table 2 and Fig. 3).
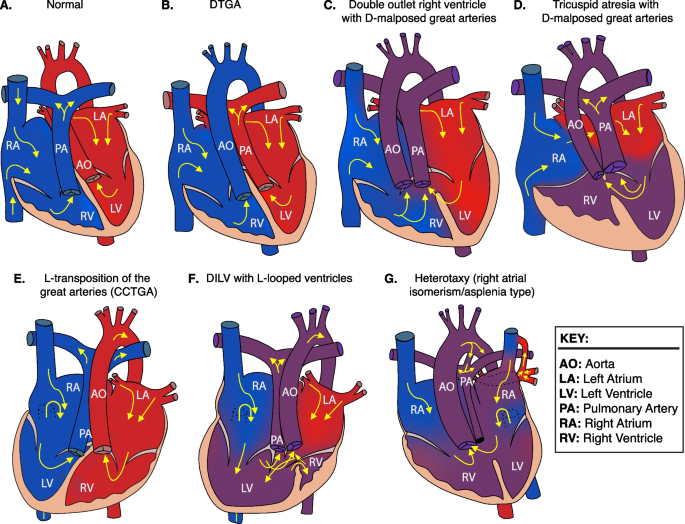
Heart depictions illustrating anatomy, blood flow (yellow arrows) and oxygenation (color coded red for oxygenated, blue for unoxygenated, and purple for poorly oxygenated) for different heart defects compared to A the normal heart anatomy. These include B dextro-transposition of the great arteries (DTGA), C double outlet right ventricle with D-malposed great arteries, D tricuspid atresia with D-malposed great arteries, E congenitally corrected transposition of the great arteries (CCTGA) with left ventricular looping and L-transposition of the great arteries, F double inlet left ventricle (DILV) with L-looped ventricles. CCTGA and DILV are illustrated with dextrocardia, but may be levocardic or dextrocardic. G Heterotaxy, asplenia syndrome/right atrial isomerism type
The case LAT0048, harboring a previously unreported p.L347F variant, presented with DTGA. Parental testing revealed that this rare variant was inherited from his father without CHD (Fig. 1). Leucine 347 is the last amino acid in the NODAL protein, and its substitution to phenylalanine is predicted to have a deleterious effect on NODAL function with a CADD score of 31 and a REVEL score of 0.51.
A recurrent indel allele potentially caused by secondary structure mutagenesis
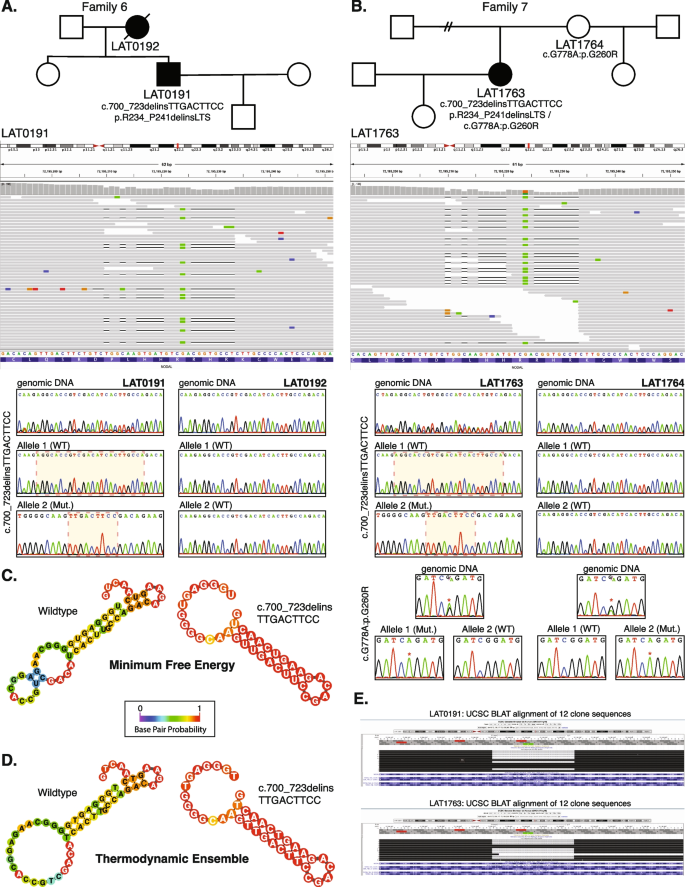
We identified one indel variant (p.R234_P241delinsLTS, c.700_723delins) in two unrelated cases (LAT0191 and LAT1763; families 6 and 7, respectively) (Table 1 and Fig. 4A, B). To facilitate variant interpretation and validation, we performed cloning experiments, to enable separation of alleles, on the probands and parental DNA for subsequent Sanger sequencing. These experiments confirmed that these two probands did not have frameshift variants, but indeed have the nonframeshift delinsLTS variant allele. Of note, the secondary structure predictions for “wild-type” (WT) and mutant single-strand nucleic acid using the RNAfold Server available at http://rna.tbi.univie.ac.at/, applying the minimum free energy (Fig. 4C) and thermodynamic ensemble (Fig. 4D) functions for intramolecular W-C base pairing, suggest that this complex mutation is mediated by secondary structure mutagenesis. The WT structure has a minimum free energy of −10.40 kcal/mol, while the mutant structure has a minimum free energy of −13.20 kcal/mol (lower by 2.80 kcal/mol). For Family 7 (LAT1763), in addition to the c.700_723delins variant allele, we also detected the c.778G > A:p.G260R variant. Allele 1 in LAT1763 is shown to be WT for the c.700_723 locus, while containing the c.778G > A variant. Allele 2 was shown to contain the c.700_723delins variant while being WT at the c.778G > A locus, confirming these variant alleles are in a trans configuration and thus represent a compound heterozygous combination of biallelic variation (Fig. 4).
Complex NODAL indel variants in two probands with congenital heart disease. A Pedigree structure for Family 6 (top) with integrative genomics viewer (IGV) view of the heterozygous complex indel variant in NODAL (below). Under the IGV view are Sanger dideoxy sequence traces for the proband, LAT0191 (left) and mother, LAT0192, (right). The Sanger sequence trace panels at top represent PCR amplification products of the variant region from genomic DNA. Individual alleles are not discernable for the proband genomic DNA trace at left. Beneath the genomic DNA results are Sanger sequence traces from cloning the amplified variant region using TA cloning kits as described in “Methods”. Top and bottom panels are representative of different populations of clones for each allele. At left, the complex c.700_723delinsTTGACTTCC, p.R234_P241delinsLTS variant is apparent in the Allele 2 (Mut) Sanger trace for the proband, LAT0191. B Pedigree, IGV view, and Sanger traces for Family 7. In addition to the c.700_723delins, p.R234_P241delinsLTS variant, Sanger traces for the nearby c.778G > A:p.G260R variant are shown. Allele 1 in LAT1763 is shown to be WT for the c.700_723 locus, while containing the c.778G > A variant. Allele 2 was shown to contain the c.700_723delins, p.R234_P241delinsLTS variant while being WT at the c.778G > A locus, confirming these variant alleles are in a trans configuration. C, D Secondary structure predictions for WT and mutant RNA using the RNAfold Server (http://rna.tbi.univie.ac.at/) using the minimum free energy (C) and thermodynamic ensemble (D) functions. Base pair probability is shown using color coding; cooler colors (blue) represent lower probability and warmer colors (red) represent higher probability. Variant RNA has more stable secondary structure as shown at right in C and D. E UCSC genome browser view of the 12 clones sequenced for LAT0191 and LAT1763, showing populations with the deletion, and with WT sequence. All variant data shown is for NODAL transcript NM_018055.5
The G260R variant observed primarily in Hispanic ancestry heterotaxy subjects
The most common variant in the study was the c.778G > A, G260R variant, detected in 17/31 CHD probands, of which all but 1 were of known Hispanic ancestry (Additional file 2: Table S7). To compare frequencies of this G260R variant allele to that observed in the “normotypical” population (https://gnomad.broadinstitute.org/variant/10-72195155-C-T?dataset=gnomad_r2_1), we focused on the largest cohort of 321 unrelated probands with laterality CHD that were recruited consecutively (Additional file 2: Table S8). Of these, 111 self-identified as fully or partially Hispanic (35%). The NODAL c.778G > A, G260R variant is by far most common in Hispanic patients, with an allele frequency of 0.00219 (76/34,592 exomes in gnomAD), with no homozygotes observed. Given this frequency, we would expect ≤ 1 G260R variant in the subcohort of 111 Hispanic laterality probands. However, we identified 10 Hispanic cases within this subcohort with a NODAL G260R variant allele (10/111 = 0.090), and two of these cases were biallelic for the G260R variant. That gives an odds ratio of 26.0 (95%CI 13.9–48.4, p < 0.0001) for the cohort. Moreover, if limiting the Hispanic subcohort to the most commonly reported NODAL-associated laterality defects (DTGA, CCTGA, DILV, other L-ventricular looping, and heterotaxy right atrial isomerism type in the cohort), 10 had the G260R variant (10/64 = 15.6%, 12/128 alleles). So, in that group of defects, the odds ratio comparing to the gnomAD population for the G260R variant is 51.4 (95%CI 27.8–95.2, p < 0.0001).
Penetrance and phenotypic variability observed with the heterozygous and homozygous G260R allele
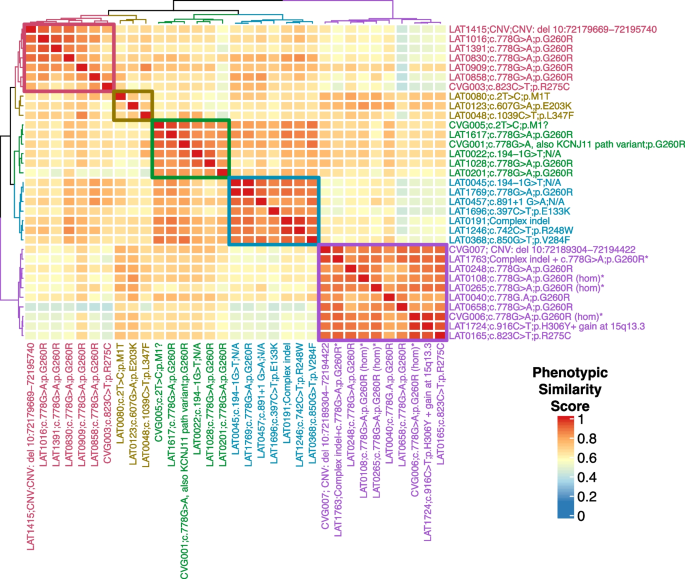
To better understand the phenotypic spectrum associated with potentially pathogenic NODAL alleles, we performed quantitative phenotypic analysis of all NODAL cases with CHD using HPO terms. However atrial septal defect, ventricular septal defect, single ventricle, and secundum atrial septal defect were excluded from the primary analysis due to the nonspecificity of these terms to rare CHD. A supplementary analysis with these terms included is provided for comparison (Additional file 3: Fig.S1). A gap statistic curve (Additional file 3: Fig.S2) was used to determine the number of groups to cluster the cases into, and a heatmap of phenotypic similarity scores and clustering was generated using HAC (Fig. 5).
NODAL proband phenotypic similarity heatmap—A heatmap was generated using proband phenotypic similarity scores and ordered based on Hierarchical Agglomerative Clustering of proband phenotypic similarity. Dendrograms showing clusters are present at the left and top sides of the heatmap. Proband IDs and variants are shown at right and bottom, and color coded based on cluster. Five clusters are highlighted by color-coded boxes on the heatmap, from top left diagonally to bottom right: red, olive, green, teal, and purple. The 4 probands with biallelic variation group in one cluster (purple box). A key for phenotypic similarity score based on color (blue corresponding to a lower score and red corresponding to a higher score) is shown at bottom right. An asterisk denotes probands with biallelic variants in NODAL
The phenotypic similarity scores among all clusters were consistently high. Notably, probands with homozygous G260R variants and the compound heterozygous case, LAT1763, formed a distinct cluster (purple group) due to a consistent phenotype: heterotaxy, right atrial isomerism/asplenia type. This group exhibited atrioventricular connection abnormalities, D-MGA, and pulmonary stenosis/atresia. A grid visualization of HPO annotated proband phenotypes (Additional file 3: Fig.S1A) highlights the severity of the phenotypic spectrum in this cluster. Heterozygous alleles displayed greater phenotypic variability across clusters (Fig. 5), with no unaffected cases reported for biallelic predicted deleterious NODAL variants, suggesting potential complete penetrance. Conversely, seven families with heterozygous G260R showed reduced penetrance (Fig. 1A, Additional file 2: Table S7).
The red and olive clusters shared higher phenotypic similarity, primarily featuring DTGA (90%) and DORV (70%) without pulmonary artery/valve atresia. The red cluster differentiated by a prevalence of straddling atrioventricular valve and hypoplastic aortic arch with coarctation of the aorta (57.1%). Notably, 66.7% of probands in the olive cluster exhibited a heterotaxy phenotype. The green and teal clusters were closely related phenotypically, with LTGA (92.3%), L-looping of the right ventricle with discordant atrioventricular connection (84.6%), and CCTGA (76.9%) as predominant features. The green cluster further stood out with pulmonary artery/valve atresia (100%) and dextrocardia as a majority feature (66.7%) (Fig. 5).
Heterozygous CNV deletions spanning NODAL in laterality defects
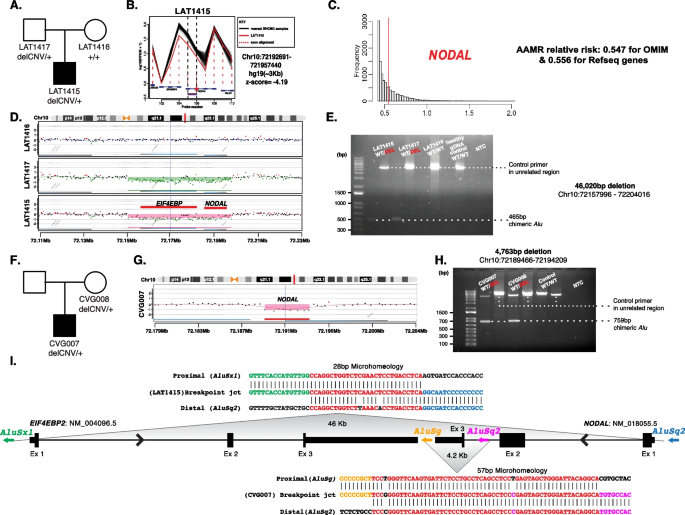
Structural genomic variation spanning NODAL was assessed in this laterality defect cohort using XHMM and HMZDelFinder CNV detection tools by comparison of ES read depth data [17, 18]. A high-confidence heterozygous deletion was observed in LAT1415 (Fig. 6A, B), who presented with tricuspid atresia, straddling mitral valve (MV), DORV, D-MGA, and severe coarctation of the aorta. ddPCR confirmed paternal inheritance, with the father having no CHD history (Additional file 3: Fig.S3).
NODAL heterozygous copy number variant (CNV) deletion alleles in laterality defect cases. A Proband LAT1415 pedigree (Family 30) (delCNV/+ heterozygous, +/+ WT homozygous). B HMZDelFinder analyses detected a CNV deletion at the 5′ start of NODAL using ES read count data (RPKM); red vertical dashed lines align to each gene exon, horizontal jagged lines (black: controls; red: deletion CNV subject) show distribution of individual read depth values for that given exon. C The Alu-Alu mediated rearrangement (AAMR) risk score for NODAL and EIF4EBP2 using AluAluCNVpredictor tool. D Family 30 aCGH showed a 46-kb deletion in proband and father. E The 1% agarose gel electrophoresis of PCR products showing the recombinant junction. The junction primer pair was designed to produce an amplicon size of 465 base pairs for deleted alleles resulting from AAMR with the formation of a chimeric Alu. F Family 31 pedigree of proband (CVG007). G Confirmation by aCGH showed a ~4 kb deletion CNV spanning NODAL exon 3. H The gel electrophoresis of PCR products showing the recombinant junction with lighter 700 base pair bands representing heterozygous deleted alleles and more intense bands (~4 kb) representing WT allele. I A schematic representation of NODAL and EIF4EBP2. Note convergent transcripts for NODAL and EIF4EBP2 (black arrow heads representing gene’s orientation). Breakpoint sequences for NODAL deletions are also shown. The proximal reference sequence and its matching proband breakpoint sequences are shown in green for LAT1415 and orange for CVG007, the distal reference sequence and its matching proband breakpoint sequences are in blue for LAT1415 and purple for CVG007, and microhomology at the junction is shown in red. The 46 kb deletion in LAT1415 presumably results from AAMR between AluSx1/AluSq2, whereas the 4 kb deletion in CVG007 proposed to result from AAMR between AluSg/AluSq2
The NODAL deletion CNV, spanning the entire gene (46 Kb), was further characterized using high-density aCGH, revealing a high instability score (0.547 for OMIM genes, 0.556 for RefSeq genes) through AluAluCNVpredictor [25], suggesting susceptibility to Alu-Alu-mediated rearrangement (AAMR; Fig. 6C). Breakpoint junction analysis (Fig. 6E) indicated direct-oriented AluSx1/AluSq2 elements, consistent with the predicted AAMR-generated event. This deletion likely resulted from a new mutation in a preceding generation within the family (Fig. 6I).
Additionally, a maternally inherited single-exon deletion of NODAL was identified in a male patient (CVG0007) with DORV, D-MGA, subaortic stenosis, and severe arch hypoplasia/coarctation (Fig. 6F–H). His mother had no CHD. This patient was recruited through BG, in which the deletion was initially predicted by CMA testing. Breakpoint junction analysis revealed an AAMR event involving directly oriented AluSg/AluSq2 (Fig. 6I).





Add Comment