Study area
We chose the Caatinga, considered the most diverse among dry tropical forests [46], due to its high frequency of chiropterophilous species, making it one of the most notable areas for such species globally [43, 47, 48]. In contrast to other forests where chiropterophilous species commonly occur in relatively low densities, the abundance of chiropterophilous species in the Caatinga facilitates the measurement of ecological processes at the population and community levels [49].
Fieldwork was conducted in the Catimbau National Park (PARNA Catimbau), located in the municipality of Buíque, State of Pernambuco (08°32′14″—08°35′12″S and 37°14′42″—37°15′02″W). The region has an average annual temperature of 25 °C and irregular rainfall, with an average annual precipitation of 700 mm. The dry season lasts for 6 to 8 months (August-February), with November being the driest month, and the rainy season concentrates from March to July, with May having the highest rainfall [50,51,52].
Studied species: guild of bat-pollinated plants
To depict the floral traits related to mechanical fit between flowers and bats, we included all the plant species occurring in the study area that are proven to be bat-pollinated, based on the list presented by Domingos-Melo et al. [43]. This allowed us to include a total of 20 species distributed among 16 genera from nine angiosperm families (Table 1). These species alternate their flowering periods throughout the year, ensuring a continuous presence of flowering plants, many of which have overlapping blooming periods (see details in Table 1). This dynamic creates a highly competitive environment among floral species for pollen transfer by bats, as all species exhibit coinciding anthesis periods. These periods begin in the late afternoon or early evening and last until early morning, with the majority of pollen being dispersed during the early hours of the night [53, 54]. For the selection of chiropterophilous plant species included in the fundamental inaccuracy, our inclusion criterion was a minimum population of 15 accessible individuals, enabling the measurement of intrapopulational phenotypic variation. The fundamental inaccuracy of each species was measured (Table 2), as well as pollen and ovule counts were carried out (Table 3). We randomly selected 20 individuals for each species, ensuring that populations of chiropterophilous plants were sampled in different locations within PARNA Catimbau (Vila do Catimbau, Pedra do Cachorro, Trilha das Torres, Alto das Torres, Pedra do Padre, Serrinha, and Alcobaça), with distances ranging from 2.0 km to 50.0 km. We ensured that all individuals were at least ten meters apart to guarantee they were not the same plant, considering that resprouting is a common behavior in the Caatinga [55].
Pollen deposition sites on bat bodies
While frugivorous bats may play a significant role as pollinators [21, 43], this study focused exclusively on the mechanical fit occurring between flowers and specialized nectar-feeding bats, namely those belonging to the subfamilies Glossophaginae and Lonchophyllinae (Phyllostomidae). In the study area, at least five bat species from these subfamilies have been recorded: Anoura geoffroyi Gray, 1838, Glossophaga soricina Pallas, 1766 (Glossophaginae), Lonchophylla inexpectata Moratelli and Dias, 2015, L. mordax Thomas, 1903, and Xeronycteris vieirai Gregorin and Ditchfield, 2005 (Lonchophyllinae) [58]. Despite having different nectar intake methods, Glossophagine and Lonchophylline bats visit flowers with similar behavior [59] as they can perform hovering flights [57, 60, 61]. These bats are highly specialized for nectar collection, characterized by an elongated snout, reduced tooth size and number, and relatively long tongues compared to their body size [60, 62]. Moreover, the notable similarity in size and body structure of these bats makes them functionally comparable in their requirements for flower-pollinator mechanical fit [21, 63]. Here, we refer to the general bauplan of visiting bats for determine pollen deposition sites. In this regard, for classification purposes, the body of the bats was divided into eigth parts, in which contact with floral reproductive structures and pollen deposition was checked: i) face, ii), head iii) neck, iv) chest, v) belly, vi) wings, vii) back, and viii) uropatagium. Additionally, we classified the way in which contact with floral reproductive structures could occur into three categories proposed by Minnaar et al. [3]: i) stroke, ii) stamp, and iii) diffuse. Stroke pollen placement requires anthers to be dragged along a part of the pollinator’s body in a consistent direction, leaving a trail of pollen. Stamp pollen placement refers to placing pollen where anthers are not dragged by the bodies of vectors but instead stamp the pollen onto the vectors’ bodies in a single contact event. Diffuse pollen placement includes any mechanisms that place pollen over large, undefined areas of vectors.
We opted not to capture bats using mist nets to collect pollen from their bodies, once as observed in the field, the grains from different body parts became mixed when the bats struggled in the net, especially pollen deposited on their wings and ventral portions. Therefore, we resorted to focal observations and photographic records for this purpose. Given that nectar-feeding bats make visits of less than a second to the flower, the description of visit details in field focal observations was limited to items such as the bat’s approach direction (frontal, from above, or below) and the manner of contact with the flower (hovering or grasping the flowers in flight).
Although bat floral visits are very quick, the specifics of the visit moment do not escape a good photographic camera lens. Therefore, the determination of pollen deposition sites on bat bodies was accomplished through photographs and videos taken with a Canon Rebel T3i camera + 70-300 mm f/4–5.6 DG Macro lens and a Sony HDR-PJ710 Handycam NightShot, respectively. The use of these images enabled the visualization of the contact location between reproductive structures and the bat’s body at the exact moment of the visit and marks of recently deposited pollen on the bat’s body (e.g., Fig. 1A, D). For each photograph in which a bat was in contact with a flower, we meticulously documented the specific points of contact between the bat and the flower’s reproductive structures. Additionally, in photographs showing the bat immediately after leaving the flower, we carefully inspected the bat’s body for pollen exhibiting the same coloration and characteristics as the flower visited. This examination aimed to identify probable deposition sites while at the same time ensuring that pollen grains from other species were not erroneously included. Only photographs that clearly displayed both the contact points and pollen deposition were considered in our analysis. Furthermore, we performed multiple independent evaluations of the photographs between authors to verify the accuracy of our observations.
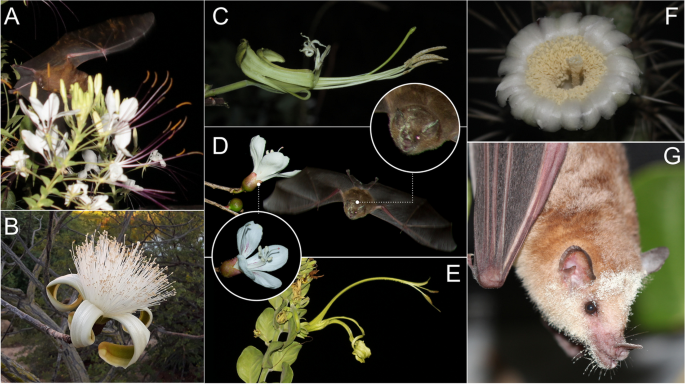
Examples of pollen placement strategies observed among chiropterophilous flowers and their pollinating bats. A Tarenaya longicarpa (Cleomaceae) flowers, with distinct yellow pollen marks are evident diffuse pollen placement on the wings of the bats; B Pseudobombax marginatum (Malvaceae) have big brush type flowers promoting diffuse pollen placement into bats. C Flowers as Bauhinia pentandra (Fabaceae) and D Ceiba gaziovii (Malvaceae) deposit pollen through unidirectional strokes, respectively in sternotribic and frontal directions. Flowers of Harpochilus neesianus (Acanthaceae) (E) and Xiquexique tuberculatus (Cactaceae) (F–G) employ a stamp-like mechanism, depositing pollen on the bats’ bodies, respectively on the uropatagium and facial regions. In photos A and B, the nectar-feeding bat Glossophaga soricina (Glossophaginae, Phyllostomidae) is shown, while photo C features Lonchophylla sp. (Lonchophyllinae, Phyllostomidae)
After careful analysis, it was possible to determine the different pollen placement strategies for each plant species. Furthermore, as several of the chiropterophilous species included in this study have previously published studies on pollination biology, information regarding the mechanical fit between flowers and bats was also obtained from them (eg. [21, 43, 53, 54, 64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].
Characterization of floral morphology
We considered various functional aspects in characterizing the flower-pollinator mechanical fit of the studied plant guild (Fig. 1). Thus, we described floral morphology through five characteristics that can summarize the main aspects important for the flower-pollinator adjustment. These are: i) floral type; ii) symmetry of vegetative whorls; iii) symmetry of reproductive whorls; iv) type of herkogamy; and v) orientation of reproductive structures. Next, we indicate how each of these characteristics was organized into different categories.
For the floral type, we used categories from classic literature on pollination biology (e.g., [56, 80]), considering general aspects of floral morphology, especially the access form to floral resources. We considered seven categories: i) brush (and pseudo-brush); ii) bell; iii) funnel/infundibuliform; iv) flag; v) dish; vi) throat; and vii) tubular.
Regarding symmetry, we based our classification on the work of Spencer and Kin [81], who categorized flowers into three groups: asymmetric (without symmetry), bilateral (zygomorphic – with a single symmetry plane), and radial (actinomorphic – with multiple symmetry planes). We decomposed symmetry into two components: the first related to vegetative whorls (calyx and corolla), mainly concerning attractiveness and the way the pollinator behaves during the visit,the second related to reproductive structures (androecium and gynoecium), determining the pollen transfer sites on the pollinator’s body.
To understand the distribution of reproductive structures within the floral space, we classified chiropterophilous flowers adapting the classification proposed by Webb and Lloyd [13]. Considering the importance of plant-animal interaction, the authors categorized herkogamy into classes that distinguish floral types based on the position of anthers and stigmas in relation to the foraging trajectory of pollinators: i) Approach herkogamy—ApH, in which the stigma is positioned in front of or above the level of the anthers, constituting the pollinator’s initial contact with the stigma; ii) Reverse herkogamy—ReH, when the stigma is positioned behind or below the level of the anthers, constituting the pollinator’s initial contact with the anthers; iii) Absent herkogamy—AbH, when anthers and stigma are at the same level and make contact with the pollinator at the same moment during its approach, or when there is no clear determination of the approach form.
In concern the orientation of reproductive structures, we used four categories: i) frontal (when reproductive organs contact the pollinator in the exact direction of its approach without directing upwards or downwards); ii) sternotribic (reproductive organs curved upwards, resulting in pollen deposition on the ventral surface of the pollinator – Fig. 1C); iii) nototribic (reproductive organs curved downwards, promoting pollen deposition on the dorsal surface of the pollinator – Fig. 1E); and iv) all (when all directions of approach are possible).
Morphometry of operational distances of floral reproductive structures
The floral morphometry of each species was conducted using flowers in early anthesis and immediately after complete opening (sample sizes are indicated in Table 2). Morphometry was performed directly in the field in some species to ensure precise measurement of the operational distances of reproductive structures since the use of fixing agents could lead to morphological alterations (e.g., Bauhinia acuruana, B. pentandra, Harpochilus neesianus, Hymenaea cangaceira) or the loss of structures sensitive to handling (e.g., Calliandra aeschynomenoides, Neocalyptrocalyx longifolium, Tarenaya longicarpa) (Table 1). For species with a linear pistils/filaments and greater resilience to handling (Dyckia spectabilis, Mimosa lewisii, Pilosocereus pachycladus and Xiquexique tuberculatus) (Table 1), their flowers were immersed in a container with 70% alcohol, and their reproductive structures were measured in the laboratory.
In each species, we measured the operational distance of the reproductive structures, defined as the linear distance from the center of the anthers (male operational distance) or the center of the stigma (female operational distance) to a landmark representing the point of access to nectar. We established a landmark for each floral type based on observations of pollinator behavior. This included considerations such as the location where the bat accesses nectar, the position of reproductive structures, and their contact with the animal’s body during flower visits. In open morphology flowers (with exposed nectar), operational distances were measured from the nectary to reproductive structures. In tubular or infundibuliform flowers, measurements were taken from the entrance of nectar chamber, as the limit of access to the bat’s snout in these flowers occurs at the entrance of the nectar chamber, where the bat inserts part of its tongue to access the resource. Digital calipers (Mitutoyo Digimatic SR44) were employed for floral measurements.
Quantification of pollen production investment
To conduct pollen counting, we collected floral buds of each species in the pre-anthesis stage (n = 5 to 10) from different individuals and preserved them in plastic containers containing 70% alcohol. For flowers with a total pollen count exceeding 2000 grains, we performed an estimation using a Neubauer chamber following standard protocols [82]. The buds were dissected in a watch glass containing a solution of 1 ml lactic acid and glycerin in a 3:1 ratio. The content was thoroughly homogenized and subsequently deposited in the Neubauer chamber using a Pasteur pipette. Pollen grains visualization and counting were conducted under an optical microscope. For species with fewer than 2000 pollen grains per flower or with large pollen grains (> 100 µm), preventing their entry into the Neubauer chamber (e.g., Bauhinia spp. and Ipomoea vespertilia), we performed a direct count of the grains on a histological slide. In species with two levels of anthers, such as Bauhinia spp, we used two of the small anthers and two of the large ones. A drop of glycerine was used for the dissection of each anther, and after homogenizing the content, we completed the process with a coverslip.
The number of ovules of each ovary was counting on a Petri dish, under a stereomicroscope (4.0 × magnification). The pollen/ovule ratio (P/O) was obtained by multiplied the pollen grains per anther, by the number of anthers in the flower, and then divided by the number of ovules. To the andromonoecious species Neocalyptrocalyx longifolium and Tarenaya longicarpa, only bisexual flowers were considered.
Statistical analyses
We compared the frequencies of pollen placement strategies employed by the studied plant guild as well as the frequencies at which different bat body parts were explored as sites of pollen transport. To do it, we ran a chi-square homogeneity test using the ‘stats’ package in R. Additionally, we plotted the proportion of species exploring each body part, along with its 95% confidence interval, and compared it with the expected proportion to identify body parts with higher or lower proportions than expected. This analysis was performed with the ‘Hmisc’ package in the R software [83].
To compare the frequencies at which various categories in each morphological floral trait occur within the guild of bat-pollinated plants under study, we employed chi-square homogeneity tests using the ‘stats’ package in R [84]. Additionally, an Analysis of Similarity (ANOSIM) was conducted on the outlined morphological floral traits, followed by Non-Metric Multidimensional Scaling (NMDS) to explore the morphospace concerning floral morphologies. Initially, ANOSIM was utilized to statistically evaluate the significance of differences between sample groups, taking into account the types of pollen placement. Subsequently, NMDS visually represented dissimilarity between samples in a reduced-dimensional space. To perform both ANOSIM and NMDS, morphological categories were transformed into dummy variables, allowing for a quantitative representation of floral attributes. The analyses were executed using the ‘vegan’ package in the R programming environment [85].
To assess differences in male and female operational distance variations across studied species, a Linear Mixed Model (LMM) was employed. The Coefficient of Variation (CV) of operational distances was used as the response variable, while sex was the predictor variable, and species was treated as a random variable. This test was conducted using the ‘nlme’ package in the R software [86].
For assessing the accuracy of each chiropterophilous species studied, we utilized fundamental inaccuracy indices based on Armbruster et al. [7]. Adaptive accuracy provides a heuristic representation of how well a phenotype aligns with its expected optimum in a population, offering insights into the level of adaptation of that phenotype. Fundamental inaccuracy indices consider only floral morphology, disregarding the pollinator’s effect on this adjustment. These indices are calculated from the means and variances of the phenotype of interest in a population, as well as its respective optimum. The formula used for inaccuracy was i = (Mf—Mo)2 + Vo + Vf, where Mf is the mean of the phenotype in question, Mo is the mean of the optimum, Vo is the variance of this optimum, and Vf is the variance of the phenotype. For comparative purposes, it is crucial to scale the obtained inaccuracy values by dividing them by the square of the mean of the analyzed phenotype (i/Mf2) [44, 45], 2009b, 2014ab). Concerning floral reproductive structures, the ideal pollen placement site on a pollinator should be related to the expected location of the pollinator with stigmas from other conspecific flowers [6]. Thus, for each species, we considered male operational distances as our phenotype of interest and female operational distances as our optimum (measured as described above) as reciprocal optima [6, 44, 87]. To assess the relative contribution of each component to the inaccuracy index, we conducted an LMM, where the percentage contributions to the accuracy index were the response variables, the components were the predictor variable, and species were treated as a random variable. This test was performed using the ‘nlme’ package in the R software. Finally, to determine if there were differences in the inaccuracy index among species with different pollen placement strategies, we conducted a Kruskal–Wallis rank sum test using the ‘stats’ package in R [84].
Regarding the investment in pollen production, we examined whether species with different pollen placement strategies differed in their total pollen production per flower and P/O ratio. For this, we conducted a Kruskal–Wallis rank sum test using the ‘stats’ package in R. We also investigated whether these attributes were related to the inaccuracy index of each species through Spearman’s rank correlation, conducted with the ‘stats’ package in R.



Add Comment