Cellular viability was not compromised following exposure
Burn pit smoke condensates at higher doses (> 50 µg/cm2) cause adverse cellular impacts in human airway epithelial cells (HAEC) [25]. To explore the impact of burn pit smoke condensate exposure gene expression and cytokine secretion from HAECs, we exposed air-liquid interface cultures of HAECs from twelve donors (Table 1) to a sub-lethal dose (25 µg/cm2) of smoke condensates from cardboard, plywood, and plastic source materials, generated under smoldering and flaming conditions, as described in the methods and reported previously [25]. Cells from equal number of donors from both sexes (female and male) were included, combining cells from both non-smokers and smokers (Table 1).
Following exposure to the burn pit smoke condensates, we first determined the viability of the cultures by measuring apical lactate dehydrogenase (LDH) secretion and transepithelial electrical resistance (TEER) of the monolayers, as surrogates of cytotoxicity detection. Previously we demonstrated that burn pit smoke condensates at the dose of 25 µg/cm2 were not cytotoxic, and cell viability was not compromised following the exposure at this dose. Similar to our previous observation, current exposure at 25 µg/cm2 did not cause any significant alteration in terms of apical LDH secretion and TEER measurements, compared to PBS-treated control group (Figure S1). These observations confirmed that the exposures used here did not cause overt cytotoxicity.
Plywood flaming condensate most effectively altered gene expression
Following exposure for 24 h to smoke condensates, we collected the cell lysate and isolated RNA to understand the potential impact of the burn pit smoke condensates on the HAEC transcriptome. Of the six condensates tested (smoldering and flaming condensates of cardboard, plywood, and plastic), flaming condensates consistently resulted in more significant DEGs compared to their smoldering counterparts (Fig. 1). The elevated numbers of significant DEGs in flaming exposure groups indicate more intensified impact of these condensates compared to smoldering ones (Fig. 1 and Table S1). Of the three waste materials tested, plywood caused the most alterations (total 329) in the HAEC transcriptome, resulting in significant up- and down- regulation of respectively 93 and 236 genes (Fig. 1 and Table S1). Flaming condensates of plastic and cardboard caused respectively 101 and 92 significant DEGs; of these DEGs, plastic flaming condensate caused 66 up- and 27 down-regulated genes (Fig. 1 and Table S1). For cardboard flaming condensate, the numbers of up- and down-regulated DEGs were 64 and 38, respectively (Fig. 1 and Table S1).
Flaming condensates caused more DEGs than smoldering ones. HAEC ALI cultures were exposed to flaming and smoldering condensates from cardboard (a), plywood (b), and plastic (c) wastes for 24 h, and modulation of gene expression was evaluated by bulk RNA sequencing. The volcano plot for each exposure group shows log2 fold changes on x-axis and log10 adjusted p-values on y-axis. Dots in the volcano plot indicate individual genes, with red color indicating significant DEGs (absolute log2FC > = 0.5 and adjusted p-value < = 0.1). Genes with the highest fold change are identified within the plot. Euler diagram comparing total significant DEGs from flaming and smoldering condensates of each waste material is shown
Compared to the flaming condensates, smoldering condensates caused fewer significant DEGs with total number for plywood, plastic and cardboard being 7, 16 and 38, respectively (Figure S2 and Table S1). Unlike flaming condensates, the proportion of significantly up- and down-regulated DEGs were almost equal for all smoldering condensates.
Pathway analysis of differentially expressed genes and the role of waste source materials
Since flaming condensates resulted in the most DEGs, we investigated any potential overlap across the three-waste materials to alter gene expression profiles. Figure 2A shows that while condensates from flaming plywood smoke caused the largest number of changes, 47 genes were commonly affected by all flaming condensates (Fig. 2a). Of these 47 genes, 18 and 29 genes were down- and up-regulated, respectively, by all flaming condensates (Fig. 2b). Gene Ontology (GO) enrichment analysis revealed that these DEGs were mostly associated with immune cell migration (leukocyte aggregation, leukocyte migration and neutrophil chemotaxis) and detoxification (toxin metabolic process, epoxygenase P450 pathway and secondary metabolic process) (Fig. 2c).
Flaming condensate modulated DEGs and pathways. DEGs from flaming condensate exposure groups were further analyzed to identify the most potent source material and commonality in gene expression modulation. Euler plot (a) showing plywood flaming condensate resulted in the most DEGs compared to cardboard and plastic. (b) Heatmap of the 47 common DEGs from all flaming condensates showing relative levels for each condensate obtained by adding the RNA-seq count values and scaling for each gene: 18 and 29 genes being respectively down- and up-resulted by all flaming condensates. Pathway analysis (c) of the 47 common DEGS exhibited impact on immune cell migration and detoxification. The color of the scale in panel B denotes relative expression level of each gene across the four groups. Scale in panel C denotes adjusted p-value
Pathway analysis of differentially expressed genes and the role of waste source materials
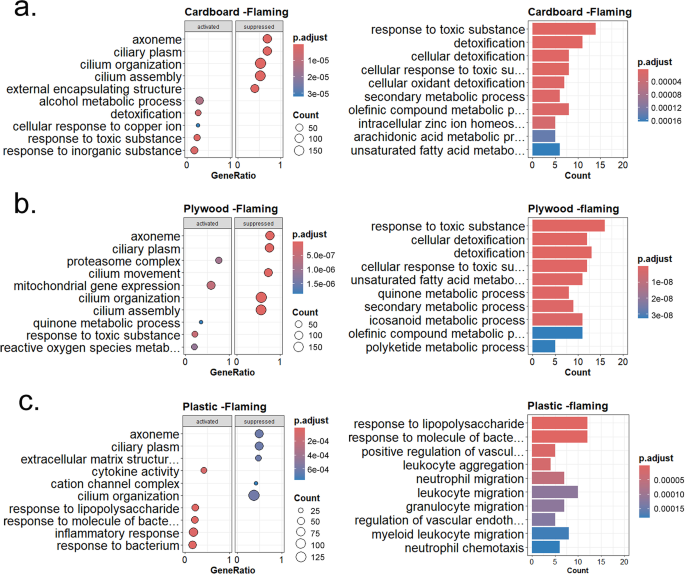
To understand the overall impact of individual waste materials on cellular functions, we performed gene set enrichment analysis (GSEA) and Gene Ontology (GO) term enrichment analyses. Figure 3 shows GSEA and GO analyses for each waste material under the flaming condition. As indicated in Fig. 3a, cardboard flaming waste exposure altered several cellular processes and molecular functions, including impairment of cilium assembly and functions and enhancement of detoxification pathways. Interestingly, genes associated with response to copper and zinc ions were elevated in HAECs exposed to cardboard flaming smoke condensate, which agrees with our previous study reporting that cardboard flaming condensate contained high levels of both copper and zinc metals [21]. When plywood flaming condensate mediated DEGs were examined by GSEA and GO enrichment analyses, similar suppression of multiciliated cell function and enhancement of detoxification pathways were noted (Fig. 3b). Several pathways associated with detoxification processes and response to reactive oxygen species were elevated in the HAECs following exposure to plywood flaming condensate.
GSEA, GO and enrichment plot of significant DEGs from flaming smoke condensate exposure. Differentially expressed genes from flaming condensate exposure groups were used to perform GSEA and GO enrichment analysis. The pairwise similarity matrix of top ten categories from GSEA is shown as the enrichment plot. The top five activated and suppressed pathways from GSEA and ten GO enrichment categories are shown as dot plot and bar diagram respectively, for cardboard (a), plywood (b), and plastic (c). The color of the scale denotes adjusted p-value, and the size of circles indicates the number of genes
In contrast to cardboard and plywood, plastic flaming condensate mostly altered genes related to leukocyte aggregation and migration. Neutrophil and granulocyte migration, and vascular endothelial growth factor (VEGF) formation dominated the DEGs created by plastic flaming condensate (Fig. 3c). The enrichment map of pairwise similarities of the enriched terms indicated that plastic flaming condensate caused activation of inflammatory processes and cellular death pathways in HAECs (Figure S3c); on the other hand, the enrichment map for plywood and cardboard flaming smoke condensate exposure groups emphasized ciliary assembly and functions (Figures S3a-b).
The smoldering combustion condition yields a reduced chemical load, as indicated by the lower levels of inorganic elements and PAHs in these condensates, compared to flaming ones [21, 23]. Accordingly, we detected a lower number of significant DEGs from the condensates generated under smoldering conditions of the three waste materials tested in the study (Figure S2). Cardboard smoldering condensate caused activation of oxidoreductase activity, especially related to pyridine cofactor nicotinamide adenine dinucleotide (Figure S4). Enrichment of pathways associated with the cell xenobiotic response were noted, with suppression ribosomal structure and subunit assembly associated genes. Plywood smoldering condensate also caused activation of oxidoreductase activity with suppression of genes associated with cell division and cell organelle lumen. In contrast, plastic smoldering condensate mediated changes in biosynthetic processes and ion (zinc and copper) regulation.
Differential gene expression is regulated by chemical components of smoke condensates
We previously detected the presence of 16 polycyclic aromatic hydrocarbons (PAHs) designated as priority pollutants by the US Environmental Protection Agency (EPA), along with several oxy/nitro PAHs and a range of inorganic elements in simulated burn pit smoke condensates [21]. To elucidate the potential mechanisms of burn pit smoke-mediated differential gene expression, we performed a correlation analysis of the 47 genes commonly modulated by the flaming condensates (Fig. 2a) with the 16 priority PAHs and 9 oxy/nitro PAHs, as described in the Methods section using Pearson’s correlation test without multiple comparison correction to perform an aggregate level of analysis. As depicted in Fig. 4, the common DEGs exhibited strong positive and negative correlation with multiple PAHs. In our present study, IL6 expression was positively modulated by all priority PAHs and six oxy/nitro PAHs (Fig. 4a). Of the 16 priority PAHs, fluoranthene, pyrene, benz(a)anthracene, chrysene, benzo(b)fluoranthene and benzo(k)fluoranthene positively and negatively modulated several DEGs. Interestingly, oxy/nitro PAHs showed strong correlation with more genes, both positively and negatively impacting the expression; as shown in Fig. 4a, apart from CRCT1, HSPA6, and CYP2A13, all other genes were either positively or negatively associated with one of four oxy/nitro PAHs (namely 9,10-anthraquinone, 1,8-naphthalic anhydride, benzanthrone and 1-pyrenecarboxaldehyde), suggesting broad impact on differential gene expression by these chemical components.
Correlation analysis between chemical components and flaming condensate induced DEGs. Pearson’s test was used to perform correlation between chemical compounds present in the condensates and the common DEGs from flaming condensate as described in the Methods section. Strong positive and negative correlation were noted for several PAHs and metals with DEGs
When the levels of inorganic elements present in smoke condensates were correlated with the 47 genes, aluminum, molybdenum, and silicon were found to be positively and negatively correlated with multiple genes (Fig. 4b). Interestingly, RASD1, ALDH3A1, VIPR1, ITGB7, CRCT1, HSPA6, CYSRT1 and RPTN genes were positively correlated with multiple elements. In contrast, VIM, SLC5A1, IGFBP5, PPP1R3C, ABI3BP, STC1, PTPRT, SCNN1G, MAB21L3 were negatively correlated with three or more elements. Although IL6 expression was positively correlated with most of the PAHs, it was only positively correlated with antimony.
Differences between demographic cohorts based on tobacco use and sex
Individual demographic attributes play a significant role in determining susceptibility to disease development and health outcomes. To understand the potential role of sex and smoking history on burn pit smoke exposure-mediated pulmonary outcome, we first determined the differences in constitutive gene expression between non-smoker and smoker donors, and between female and male donors, as detailed in the Methods section. To do so, we compared the gene expression in vehicle-treated cells from the groups and determined change in gene expression pattern between the groups. Significant DEGs comparing non-smokers with smokers, and female and male donors are reported in table S2. As shown in Figure S5a, multidimensional scaling (MDS) plot of non-smokers and smokers’ samples did not indicate any specific clustering. A total of seven significant DEGs were detected. Importantly, gamma ENaC subunit expression was higher in smokers (Figure S5b), indicating that smokers may be susceptible to increased airway dehydration.
Previous studies have reported different gene expression in airway cells from females and males [46, 47]. When gene expression pattern in HAECs from female and male donors was compared, the MDS plot clearly indicated clustering of the samples based on sex with minimal overlap (Fig. 5a). We identified a total of 59 significant DEGs between HAECs from female and male donors, 9 and 50 of these genes were respectively up- and down-regulated in female donors, compared to the male donors (Fig. 5b). Interestingly several of these DEGs have already been reported by other groups. As expected, the long noncoding RNA X-linked X-inactive-specific transcript (XIST), which plays a critical role in X-chromosome inactivation in females [48], was elevated in female donors compared to male donors (Fig. 5a). Similar to previous reports [46, 47], ZFX, RPS4X and KDM6A were also increased in female donors. Conversely, RPS4Y1, KDM5D, USP9Y and DDX3Y were elevated in male donors, as previously reported [49]. Several genes coding for kinesin superfamily proteins (KIFs: KIF11, KIF2C, KIFC1, KIF4A and KIF20A), cyclin protein coding cell cycle-related genes (CCNA2, CCNB1, and CCNB2) and ubiquitin-conjugating enzyme genes (UBE2S, UBE2T, and UBE2C) were elevated in the male donors. Sex chromosome-encoded RNA helicases DDX3X and DDX3Y were respectively elevated in female and male donors (Fig. 5b).
Background differences in gene expression profiles between biological female and male donors. Baseline gene expression pattern between female and male donors were distinctly different, as identified in the multidimensional scaling (MDS) plot (a). A total 59 DEGs were noted between female and male; heatmap (b) showing the normalized level of each gene in individual donor including 9 up- and 50 down-regulated genes in female donors. The top ten pathways activated and suppressed in female donors (c), as identified by GSEA, are shown as a dot plot
GSEA and GO enrichment analysis were utilized to understand the differences between female and male donors. As shown in Fig. 5c, genes associated with vascular structure were activated in HAECs from female donors; on the other hand, genes related to multiciliated cell structure and function were suppressed in female donors. The GO enrichment pathways in female and male donors’ cells are shown as Figure E6 indicating categories significantly associated with the DEGs.
Demographic characteristics and the effects of simulated burn pit smoke condensate exposure
To evaluate any donor demographic-based differential impact of burn pit smoke condensate, we estimated the significant DEGs separately among cells derived from non-smokers, smokers, female, and male donors (six in each cohort). When flaming condensate derived significant DEGs were considered in total, smokers cells exhibited more alterations compared to non-smoker donor cells (Fig. 6a, Tables S3 and S4). Following exposure to flaming condensates cells from donors without any reported smoking history showed a reduced number of DEGs compared to HAECs from donors with a smoking history (Fig. 6a). For cardboard flaming condensate exposure, these cells showed a total of 155 DEGs (Table S4; 81 up and 74 down) compared to 52 DEGs (Table S3; 27 up and 25 down) in HAECs from non-smoking donors. When plastic flaming smoke condensate mediated DEGs were compared between non-smokers and smokers, cells from the latter exhibited more DEGs (Table S4, total 148; 100 up and 48 down) compared to the former group (Table S3, total 30; 16 up and 14 down). As noted earlier, plywood flaming condensate exposure results in the highest numbers of significant DEGs; HAECs from donors with smoking history showed 484 DEGs (Table S4; 133 up and 351 down) compared to HAECs from non-smoking donors (Table S3, total 225; 68 up and 157 down).
Distinct role of the host factors flaming burn pit smoke condensate exposure. Donors were separated based on the smoking history (non-smokers and smokers) and biological sex (female and male) and Euler diagrams were generated using significant DEGs by flaming smoke condensate was evaluated as described in the Methods. (a) All three flaming condensates caused more alterations in cells from donors with history of smoking than non-smoker donors. Comparison based on sex (b) demonstrated that female donors’ cells were more responsive to flaming condensate exposure than male donors’ cells
Comparison based on the sex of the donor following exposure to flaming condensates showed more alterations in HAECs from female donors than male donors (Fig. 6b). Cardboard flaming condensate caused 139 (Table S5; 70 up and 69 down) and 43 (Table S6; 29 up and 14 down) DEGs in respectively HAECs from female and male donors. Plastic flaming condensate also caused more DEGs in HAECs from female donors with a total number of significant DEGs of 147 (Table S5; 90 up and 57 down) than HAECs from male donors (Table S6, total 32; 21 up and 11 down). Flaming condensate of plywood waste caused a total 459 DEGs (Table S5; 132 up and 327 down) in HAECs from female donors versus a total of 225 in cells from male donors (Table S6, 69 up and 156 down).
Compared to the flaming condition, smoldering burn pit smoke condensates caused lower numbers of DEGs (Figure S7); hence, a demographics-based comparison was mostly inconclusive for these exposure groups. The only impact of donor’s smoking history on condensate derived DEGs was noted for cardboard smoldering condensate exposure, where HAECs from donors with smoking history showed a total 81 DEGs (Table S4; 33 up and 48 down) compared to cells from non-smokers (Table S3, total 20; 3 up and 17 down). A marginally elevated number of DEGs was noted in cells from non-smokers following plastic condensate exposure (Table S3, total 24; 4 up and 20 down) as compared to HAECs from donors with a smoking history (Table S4, total 17; 15 up and 2 down). Sex-based comparison of DEGs from smoldering condensates only showed more DEGs in HAECs from male donors (Table S6, total 58; 12 up and 46 down) following cardboard condensate exposure than HAECs from female donors (Table S5, total 42; 22 up and 20 down).
To understand the susceptibility of the four demographic cohorts towards burn pit smoke exposure-derived diseases, we used significant DEGs from plywood flaming smoke condensate exposure group to perform enrichment analysis, utilizing a gene-disease association database from DisGeNET [41]. Both up- and down-regulated genes were used to perform enrichment analysis of disease gene signatures. Table S7 reports the results of enrichment analysis, including significantly associated diseases in the four demographic cohorts. Figure S8 demonstrates the human diseases associated with the four demographic cohorts. All of the cohorts showed a significant association with contact dermatitis, contact hypersensitivity, and cancer of different organs. When focusing on respiratory diseases, HAECs from non-smokers exhibited association with asbestosis and asbestos exposure-derived pulmonary fibrosis. Alterations in gene expression in HAECs from donors with a smoking history were closely associated with asthma, bronchiectasis, and emphysema. When sex-dependent associations were examined, female donors were associated with asthma, several types of neoplasm and carcinoma.
Co-modulation of cytokine secretion by smoke condensates
We have previously shown that exposure to burn pit smoke condensate causes modulation of cytokine secretion by HAECs [25]. When the basolateral media of the HAECs was analyzed for the secretion of cytokines, we observed that eleven (IP-10, IL-7, IFN-g, MCP-1, IL-1b, Eotaxin-3, IL-1a, IL-12p70, GM-CSF, IL-6, VEGF-A) of the total seventeen cytokines were significantly altered by condensate exposures compared to vehicle control (Table S8). However, pairwise comparison did not identify many significant differences (Table S8). The relative levels of each cytokine across the different exposure groups are shown as a heatmap (Fig. 7a). To further emphasize the co-modulation of the cytokines by burn pit smoke condensates, we used data collapsing approaches to identify clusters of mediators that are likely co-modulated, similar to our previous studies [32]. The vehicle control group is divided into cohorts representing samples from four demographic groups: non-smokers, smokers, female and male. Three clusters of co-modulating cytokine mediators were detected, which included respectively seven, six and four mediators (Fig. 7b). Cluster 1 included most chemokines (Eotaxin-3, MCP-1 and TARC), gamma-chain cytokines (IL-2, and IL-7), IL-10 and VEGF-A; cluster two included six mediators (IL-1α, IL-1β, IFN-γ, IL-8, IL-6, and IL-12p70), including mostly pro-inflammatory cytokines; cluster three included four mediators (GM-CSF, IL-13, IP-10, and TNF-α) with both pro- and anti-inflammatory properties. When individual cluster modulation was considered, plywood flaming condensate caused the most suppression of clusters one and three, with the highest activation of cluster two (Fig. 7c). Flaming condensate of cardboard induced cytokines belonging to the cluster one. These observations suggest that burn pit smoke condensate-mediated modulation of cytokine secretion by HAECs is source material and combustion temperature dependent, and plywood flaming smoke condensate induces the highest immunomodulatory/inflammatory response in HAECs.
Co-modulation of cytokines by smoke condensates. Basolateral media was collected following burn pit smoke condensate exposure and HAEC-secreted mediators were quantified by MSD analysis. Seventeen mediators were further analyzed using WGCNA method and grouped into three clusters (a) based on co-modulation in control samples. Relative impact of smoke condensates on each cluster was evaluated as described in the Methods; bar graph (b) shows the relative score representing modulation of each cluster by the condensates











Add Comment