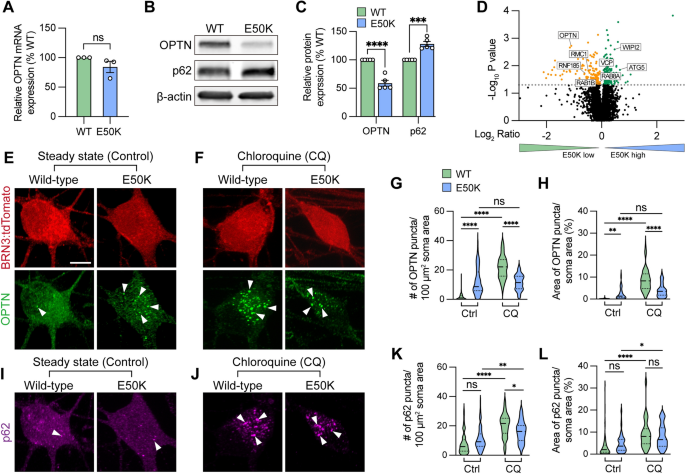
The glaucoma associated OPTN(E50K) mutation alters endogenous protein modification and leads to protein accumulation in hPSC-RGCs
We have previously established a human patient-specific induced pluripotent stem cell (iPSC) line with an OPTN(E50K) mutation, as well as an isogenic control cell line through the CRISPR/Cas9 correction of the mutation. Similarly, we generated a second isogenic pair by introducing the OPTN(E50K) mutation into an unaffected H7 hPSC line carrying an RGC-specific BRN3b-tdTomato-Thy1.2 reporter [12]. Both OPTN(E50K) lines exhibited neurite retraction and hyperexcitable phenotypes [12], although the mechanisms leading to these changes were unclear. In the current study, we approached each set of analyses with these two isogenic pairs of cell lines including OPTN(E50K) and unaffected control (wild-type) RGCs to minimize genetic variability among comparisons, and analyses were typically performed at stages of RGC maturation at which they had been previously demonstrated to exhibit disease-associated phenotypes [12].
To study how the endogenous OPTN(E50K) mutation attenuates RGC homeostasis, we first characterized the expression of OPTN. While the level of OPTN mRNA was not significantly changed among wild-type and OPTN(E50K) RGCs, there was a 41.1 ± 5.2% (mean ± SEM.) reduction in the level of OPTN protein in RGCs with the OPTN(E50K) mutation, along with a 28.1 ± 4.3% increase in the autophagy receptor p62 (Fig. 1A–C), consistent with previous findings that the E50K mutation decreased the overall abundance of OPTN protein in the mouse eye [40]. To verify these results, we also referenced our previously obtained RNA-seq data [12], which confirmed the lack of changes in expression at the transcriptional level observed in our qRT-PCR results. These results indicated that the E50K mutation likely altered OPTN protein expression through post-translational modifications, and the increased level of p62 may be associated with autophagic accumulation. To ensure that this difference in OPTN protein was not due to changes in solubility, we performed Western blots of both the soluble and insoluble fractions, which both demonstrated significant decreases in OPTN protein (Figure S1). To rule out the possibility that the reduction of OPTN protein was due to a decrease in the specificity of the antibody due to the E50K mutation, we performed an unbiased proteomics analysis of isogenic control and OPTN(E50K) RGCs two weeks after purification and identified 154 downregulated proteins as well as 178 upregulated proteins (Fig. 1D). Among the downregulated proteins, OPTN was identified with 4 peptides and 5 peptide-spectrum matches (PSM), and levels were significantly decreased in OPTN(E50K) RGCs, corroborating our Western blot results. Of interest, we identified an additional 7 autophagy-associated proteins whose expression was also altered, suggesting further disruption of the autophagy pathway due to the OPTN(E50K) mutation (Fig. 1D). Interestingly, proteomics data also indicated misregulation of several ubiquitination-associated proteins, including USP14, UBE2D1, ARIH1, HUWE1, UBA52, and BRCC3, while analyses of Biological Processes demonstrated a misregulation of proteins involved in processes such as protein transport, mRNA processing, and autophagy, while Pathway Analyses suggested changes associated with numerous neurodegenerative diseases (e.g. ALS, Parkinson’s, Huntington, etc.) as well as changes in pathways associated with the proteosome and spliceosome (Figure S2). These analyses of proteomics results also demonstrated the misregulation of numerous proteins associated with mitochondrial or endoplasmic reticulum function, providing the possibility that some differences in OPTN protein expression may be associated with changes in cellular processes such as ubiquitination and subsequent processing, as well as autophagy and mitophagy.
The glaucoma associated OPTN(E50K) mutation alters endogenous protein modification and aggregates in hPSC-RGCs. A Real-time RT-PCR quantification of OPTN mRNA levels (n = 3 for each WT and E50K; t-test, p = 0.181). B–C Western blot and the relative protein expression of OPTN and p62 to β-actin in hPSC-RGCs (n = 5 for each WT and E50K; t-test, OPTN ****p < 0.0001, p62 ***p = 0.0002). D Proteomics analysis demonstrated changes in the expression of autophagy-associated proteins in OPTN(E50K) hPSC-RGCs. E–F Immunostaining displayed the expression of OPTN puncta in BRN3:tdTomato hPSC-RGCs from WT and E50K under steady state (control) and chloroquine (CQ) treatment. Arrows indicate the OPTN puncta. Scale bar: 10 μm. G–H Quantification of OPTN puncta in hPSC-RGCs. I–J Immunostaining displayed the expression of p62 puncta in BRN3:tdTomato hPSC-RGCs from WT and E50K under control and CQ treatment. K–L Quantification of p62 puncta in hPSC-RGCs. G–H and K–L: n = 3 biological replicates using Ctrl-WT n = 60, Ctrl-E50K n = 60, CQ-WT n = 57 and CQ-E50K n = 48 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). Data are represented as mean values ± SEM
Under homeostatic conditions, the number of autophagosomes is balanced between autophagic biogenesis and degradation by the lysosome. Wild-type RGCs showed a predominantly cytosolic OPTN pattern with few puncta observed in RGCs (Fig. 1E), similar to patterns observed previously [41]. However, we observed that OPTN(E50K) RGCs displayed a significant increase in OPTN puncta within RGC somas, indicating an abnormal phenotype (Fig. 1E). To measure any preliminary changes in autophagic biogenesis without the loss of any autophagosomes due to lysosome-mediated degradation, we inhibited autophagosome-lysosome fusion by treatment with chloroquine for 16 h prior to fixation, as previously described [42], where we found that the number of OPTN puncta were significantly decreased in OPTN-E50K RGCs compared to control RGCs following chloroquine treatment (Fig. 1F). More intriguing, however, were the comparisons made not only between control and OPTN-E50K RGCs within each treatment group, but those comparisons across treatment groups (Fig. 1G–H). Indeed, the treatment of control RGCs significantly increased the number of OPTN puncta, supporting the idea of chloroquine inhibiting autophagic flux. However, when OPTN-E50K RGCs were treated with chloroquine, there was no significant change in the number of OPTN puncta observed compared to untreated OPTN-E50K RGCs, supporting the idea that the OPTN-E50K mutation itself either already inhibited autophagic flux or altered OPTN protein structure that accumulated under homeostasis condition (Fig. 1E–H). Additionally, no difference was observed in another autophagy receptor p62 puncta abundance between wild-type and OPTN(E50K), as the puncta were significantly increased after chloroquine treatment both wild-type and OPTN(E50K) RGCs, indicating that the E50K mutation did not affect p62 protein processing (Fig. 1I–L). To rule out the possibility that the genomic background of the cell line caused this phenotype, including silent mutations introduced during CRISPR/Cas9 genome editing [12], we performed the same experiments and observed the same trends in RGCs derived from another OPTN(E50K) iPSC line in comparison with its genome-corrected isogenic control (Figure S3). Collectively, these findings suggest that the E50K mutation adversely affected RGCs through the accumulation of OPTN protein, potentially contributing to RGC neurodegeneration.
OPTN(E50K) hPSC-RGCs display autophagosome accumulation and impaired autophagic-lysosomal degradation
During the process of autophagy, OPTN is necessary to recruit the microtubule-associated protein light chain 3 (LC3) for the engulfment of protein aggregates and/or damaged organelles by formation of the autophagosome [29, 43]. To examine whether this OPTN functional recruitment changes due to the E50K mutation in RGCs, we first used a GFP-fused LC3 reporter to visualize the co-localization of LC3 and OPTN. We found that 10.7 ± 1.2% of LC3 puncta co-localized with OPTN in wild-type RGCs, while only 5.1 ± 0.6% co-localized in RGCs with the OPTN(E50K) mutation (Fig. 2A–I), suggesting that the E50K mutation attenuated OPTN recruitment of LC3. Inhibition of lysosome-mediated degradation by chloroquine demonstrated a similar outcome (Figure S4). We observed no difference in the cytosolic LC3-I level in OPTN(E50K) RGCs (Fig. 2J, K), but a significant increase in the lipidated form of LC3-II, an indicator of autophagosome abundance [44], as well as autophagic flux as determined by the ratio of LC3-II/LC3-I (Fig. 2J–L). Importantly, lysosomal protein LAMP1 expression was also upregulated in OPTN(E50K) RGCs (Fig. 2J, K), suggesting that the OPTN(E50K) mutation not only induced autophagosome accumulation but also changed lysosomal degradation.
OPTN(E50K) hPSC-RGCs displays autophagosome accumulation and impairs autophagic-lysosomal degradation. A–I Analysis of protein colocalization between OPTN and LC3 in hPSC-RGCs (n = 3 biological replicates using WT (n = 36) and E50K (n = 40); t-test, ****p < 0.0001). Yellow arrows label the colocalization between OPTN and LC3 puncta, and white arrows represented that the LC3 puncta did not colocalize with OPTN. Scale bar: 10 μm. J–L Western blot and subsequent analysis of relative protein expression demonstrated increased LC3-II, LAMP1 and autophagic flux (LC3-II to LC3-I ratio) in OPTN(E50K) hPSC-RGCs (n = 6 for each WT and E50K; t-test, LC3-I p = 0.41, LC3-II *p = 0.016, LAMP1 **p = 0.0018, LC3-II/I *p = 0.039). M Schematic of LC3-RFP-GFP probe paradigm. N–V Using LC3-RFP-GFP probe showed the accumulation of autophagosome (RFP + GFP +) in OPTN(E50K) hPSC-RGCs (n = 3 biological replicates using WT n = 44 and E50K n = 45 technical replicates; t-test, **p = 0.0075). Scale bar: 10 μm in A, also refers to E; 5 μm in B, also refers to C, D, F–H. Data are all represented as mean values ± SEM
Disruption of the autophagic-lysosomal pathway has been implicated in multiple neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis, leading to a deficit in protein degradation [45,46,47]. Since we observed the upregulation of autophagosome and lysosome proteins in OPTN(E50K) RGCs, we investigated whether these changes occurred in response to perturbations in the autophagic-lysosomal pathway by expressing RFP and GFP fused to LC3 in RGCs (Fig. 2M) [48]. This probe discriminated between the autophagosome and the acidic autolysosome due to differences in pH sensitivity. Both RFP and GFP signals were expressed in the autophagosome, while under acidic conditions within the autolysosome, the RFP signal persisted as the GFP signal was extinguished. As our established hPSC-RGC system previously included a BRN3-tdTomato reporter, whose red fluorescence could interfere the results using this probe, we used CRISPR/Cas9 genome editing to insert the OPTN(E50K) mutation in the H7 hPSC line without the BRN3-tdTomato reporter, and subsequently identified RGCs by staining with an antibody against BRN3 when imaging. In wild-type RGCs, 25.5 ± 2.8% of RFP puncta co-expressed GFP, indicating the remaining 74.5 ± 2.8% of autophagosomes had fused with the lysosome (Fig. 2N–Q, Supplemental Movie S1). However, OPTN(E50K) RGCs exhibited significantly more overlap between RFP and GFP at 35.8 ± 2.5% (Fig. 2R–V, Supplemental Movie S2) and increased the number of GFP puncta but not RFP (Fig. 2V), highlighting that the OPTN(E50K) mutation resulted in an impaired ability of the autophagosome to properly fuse with the lysosome for subsequent degradation.
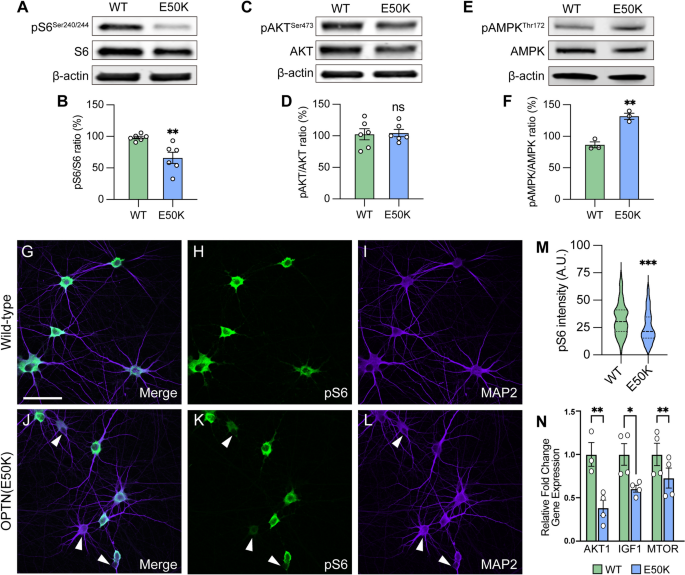
OPTN(E50K) hPSC-RGCs downregulate mTORC1 signaling
The mammalian target of rapamycin (mTOR) signaling pathway is a key metabolic regulator and sensor of stress. Activation of mTOR is known to promote dendritic morphological complexity and axonal regeneration in RGCs, while also functioning as a negative regulator of autophagy through the mTORC1 complex [49,50,51,52,53]. Under cellular stress in neurons, autophagy disruption can activate adenosine monophosphate-activated protein kinase (AMPK) to induce the repression of mTORC1, resulting in neurodegeneration [54, 55]. To determine whether autophagy disruption due to the OPTN(E50K) mutation induced RGC degeneration associated with mTORC1 signaling, we used pS6Ser240/244 as a readout of mTORC1 activity [56]. We first characterized mTORC1 activity in hPSC-derived retinal cells by isolating cells from retinal organoids after 50 and 80 days of differentiation, respectively, which allowed for the analysis of the majority of neuroretinal cell types including RGCs (BRN3B-tdTomato), retinal progenitors (CHX10), and photoreceptors (OTX2) (Figure S5A–C). Immunocytochemical detection of pS6Ser240/244 revealed a robust expression within BRN3B:tdTomato RGCs, but not in retinal progenitor cells nor photoreceptors, indicating a strong role for mTOR signaling specifically within hPSC-RGCs (Figure S5D, E). Subsequently, we analyzed RGCs that were isolated from retinal organoids and allowed to mature for an additional 4 weeks, a timepoint at which we have previously demonstrated to result in neurodegenerative phenotypes in OPTN(E50K) RGCs [12]. OPTN(E50K) RGCs exhibited a decreased expression of the mTORC1 effector pS6Ser240/244, while no difference was observed in the expression of the mTORC2 effector pAKTSer473 (Fig. 3A–D). More so, OPTN(E50K) RGCs also increased the level of the phosphorylated form of AMPK (pAMPKThr172), which is a stress activator and mTORC1 inhibitor (Fig. 3E, F). Immunocytochemistry also allowed for the more detailed investigation of pS6Ser240/244 within individual RGCs, which revealed a profound decrease in the expression of pS6Ser240/244 intensity in the somatic area of a subset of OPTN(E50K) RGCs (Fig. 3G–M), while others appeared to maintain normal expression levels. To further explore changes in mTOR signaling as a result of the OPTN(E50K) mutation, we then explored prior RNA-seq data comparing isogenic control and OPTN(E50K) RGCs taken after just 10 days of maturation [12], and found a significant reduction in the expression of some other mTOR-associated genes (Fig. 3N). Taken together, our results suggest that chronic autophagy deficits in glaucomatous RGCs were also associated with the activation of AMPK signaling to suppress mTORC1.
Attenuation of mTORC1 signaling in OPTN(E50K) hPSC-RGCs. A–F Western blot and the relative protein expression of pS6Ser240/244, pAKTSer473 and pAMPKThr172 to its total protein, respectively, in hPSC-RGCs (n = 6 for each WT and E50K; t-test, pS6Ser240/244 **p = 0.0057; pAKTSer473 ns = not significant, p = 0.854; pAMPKThr172 **p = 0.0027). G–M Immunostaining and quantification of pS6Ser240/244 intensity revealed that a subset of RGCs with the OPTN(E50K) mutation reduced the expression of pS6Ser240/244 (n = 3 biological replicates using WT n = 96 and E50K n = 96 technical replicates; t-test, ***p = 0.0009). Arrows indicate RGCs that have reduced expression of pS6Ser240/244. N RNA-seq analyses demonstrated a significant reduction in some mTOR-associated genes. Scale bar: 25 μm. Data are all represented as mean values ± SEM
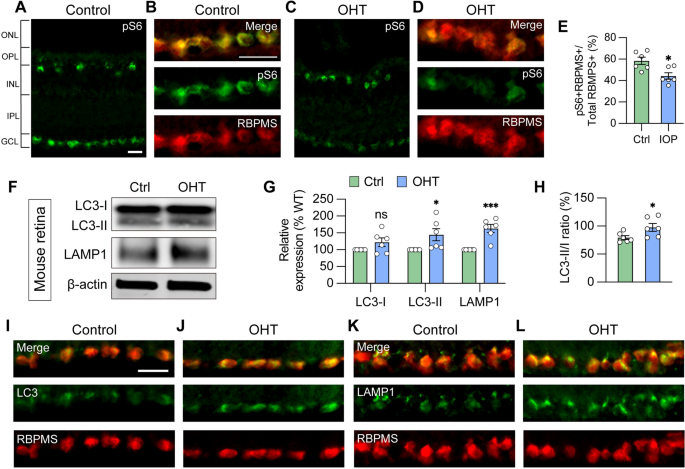
Glaucoma RGCs subjected to ocular hypertensive stress demonstrated autophagy deficits similar to OPTN(E50K) RGCs
Many underlying causes of glaucoma may exist, including elevated intraocular pressure, environmental factors, and genetic contributions. Despite varying underlying causes, pathological features are often similar across patients, suggesting perhaps that some cellular mechanisms may be consistent. Multiple missense mutations in OPTN or TBK1, known as autophagy regulators, are known to result in normal tension glaucoma [27, 57], suggesting that autophagy homeostasis plays a key role in RGC survival and, conversely, inappropriate autophagy processing may contribute to glaucomatous neurodegeneration. However, it was unclear if the phenotypes observed in OPTN(E50K) RGCs thus far were relevant to other forms of glaucoma. Thus, to determine if the dysfunctional autophagy phenotypes observed in OPTN(E50K) RGCs were similarly identified in other models of glaucoma, thereby suggesting a broader relevance in RGC neurodegeneration, we used a well-established magnetic microbead occlusion glaucoma model to induce ocular hypertension through the injection of magnetic microbeads into the anterior chamber of the mouse eye. This procedure blocks aqueous humor outflow resulting in elevated intraocular pressure (IOP) (Figure S6 A) [37, 58], a major risk factor for the development of glaucoma. Indeed, RGCs from sham-injected control mice exhibited robust mTORC1 activity, as visualized by pS6Ser240/244 co-localization with the RGC marker RBPMS, while decreased mTORC1 activity was observed in RGCs subjected to ocular hypertensive stress (Fig. 4A–E). This observation is in agreement with previous findings that mTORC1 signaling is partially inactivated in glaucomatous RGCs through AMPK phosphorylation resulting in dendritic retraction [58], and also corroborates results obtained with OPTN(E50K) RGCs (Fig. 3G–M).
Glaucoma RGCs subjected to ocular hypertensive stress demonstrate autophagy deficits. A, B Immunostaining labeled the level of the mTORC1 effector pS6Ser240/244 and exhibited robust activity in the ganglion cell layer (GCL), co-labeled with RBPMS in B, and inner nuclear layer (INL) in control mouse retina. Scale bar: 25 μm. C–E Under ocular hypertension, immunostaining and associated quantification demonstrated that the level of pS6Ser240/244 decreased in mouse RGCs (n = 3 mice/group, 2 images/mice; t-test, *p = 0.011). F–H Western blot verified changes in protein expression of LC3-I, LC3-II, LAMP1, and LC3-II/I ratio in control or glaucoma mouse retinas with ocular hypertension (OHT) (n = 6 for each control and OHT; t-test, LC3-I p = 0.122, LC3-II *p = 0.034, LAMP1 ***p = 0.0004, LC3-II/I *p = 0.042). I–L Immunostaining displayed the elevation of LC3 and LAMP1 in RBPMS-expressing RGCs after ocular hypertension. Scale bar: 25 μm. Data are all represented as mean values ± SEM
Subsequently, changes in the expression of autophagy markers were examined 2 weeks after microbead injection, a time that precedes RGC loss and thereby avoids the confounding effect of overt neurodegeneration [58]. A significant increase in LC3-II, the LC3-II/I ratio, and LAMP1 were observed in the retina 2 weeks after ocular hypertension induction compared to sham-injected controls (Fig. 4F–H), in agreement with our results obtained with OPTN(E50K) hPSC-derived RGCs (Fig. 2J–L). Immunostaining of retinal sections further showed the increased expression of LC3 and LAMP1 more specifically in the ganglion cell layer, which co-localized with the RGC marker RBPMS (Fig. 4I–L), similar to results observed by others in mouse models [24]. No changes were observed in the expression of OPTN protein within the OHT and control mouse retinas as determined by Western blot (Figure S7), although this lack of difference could be due to the analysis of total retinal protein rather than purely RGC protein content. Collectively, our findings highlighted that autophagy disruption is associated with RGC neurodegeneration not only in hPSC-RGCs with the OPTN(E50K) mutation, but in an ocular hypertension system, suggesting that autophagy disruption in RGCs may be a common mechanism that contributes to neurodegeneration across multiple glaucoma models.
Selective degeneration of RGCs with the OPTN(E50K) mutation
To determine if the OPTN(E50K) mutation selectively promotes neurodegeneration in RGCs as indicated by its role in glaucoma, or if it can confer effects within other cell types in an in vitro model, we evaluated the effects within two types of neurons– hPSC-RGCs and hPSC-cortical neurons (Figure S6 B), based upon their neurite outgrowth and protein expression profiles, respectively. We confirmed that wild-type and OPTN(E50K) cells were both effectively differentiated from hPSCs in parallel cultures based upon the expression of lineage-associated makers, including of BRN3/MAP2 (RGCs), and CTIP2/bIII-tubulin (cortical neurons) (Fig. 5A, D, respectively). RGCs with the OPTN(E50K) mutation exhibited shorter neurites compared to wild-type, as analyzed by neurite complexity, soma area, number of primary neurites, and total neurite length (Fig. 5 A–C, G–I), concomitant with our previous findings [12]. In contrast, neurites from cortical neurons did not exhibit any significant differences between wild-type and OPTN(E50K) under the same measured parameters (Fig. 5D–I), indicating that neurodegenerative features were selectively identified in RGCs with the OPTN(E50K) mutation.
Selective degeneration of RGCs with the OPTN(E50K) mutation. A Immunostaining to characterize wild-type and OPTN(E50K) RGCs. Scale bar: 25 μm. B Representative neurite tracing of wild-type and OPTN(E50K) RGCs after 4 weeks of purification. Scale bar: 150 μm. C Sholl analysis revealed the neurite complexity in wild-type and OPTN(E50K) RGCs (n = 3 biological replicates using WT n = 15 and E50K n = 17 technical replicates; t-test, ****p < 0.0001). D Immunostaining to characterize wild-type and OPTN(E50K) cortical neurons. E Representative neurite tracing of wild-type and OPTN(E50K) cortical neurons 4 weeks after purification. Scale bar: 150 μm. F Sholl analysis revealed similar neurite complexity in wild-type and OPTN(E50K) cortical neurons (n = 3 biological replicates using WT n = 16 and E50K n = 17 technical replicates; t-test, ns = not significant, p = 0.37). G–I Quantitative analysis of neurite parameters displayed neurite deficits in OPTN(E50K) RGCs based upon measurements of soma area (n = 3 biological replicates using RGC-WT n = 101, RGC-E50K n = 112, cortical-WT n = 68, and cortical-E50K n = 66 technical replicates; t-test, RGC: *** p = 0.0003; cortical: ns = not significant, p = 0.811) G, number of primary neurites (n = 3 biological replicates using RGC-WT n = 15, RGC-E50K n = 17, cortical-WT n = 16, and cortical-E50K n = 17 technical replicates; t-test, RGC: ** p = 0.004; cortical: ns = not significant, p = 0.626) H and total neurite length (n = 3 biological replicates using RGC-WT n = 15, RGC-E50K n = 17, cortical-WT n = 16, and cortical-E50K n = 17 technical replicates; t-test, RGC: **** p < 0.0001; cortical: ns = not significant, p = 0.575) I, but not in OPTN(E50K) cortical neurons when compared with wild-type. J–M Western blot and quantified relative protein expression demonstrated that the OPTN(E50K) mutation altered LC3-II only in RGCs (n = 3 for each WT and E50K from RGC or cortical neurons; One-way ANOVA, Tukey post hoc test. ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). N–U Immunostaining displayed the expression of OPTN puncta in hPSC-derived cortical neurons from WT and E50K under steady state (control) N–Q and chloroquine (CQ) treatment R–U. Scale bar: 10 μm. V, W Quantification of OPTN puncta in hPSC-derived cortical neurons (n = 3 biological replicates using Ctrl-WT n = 39, Ctrl-E50K n = 34, CQ-WT n = 37 and CQ-E50K n = 37 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, *p < 0.05, ns = not significant, p > 0.05). Data are all represented as mean values ± SEM
We then sought to determine how the OPTN(E50K) mutation affected the expression of some autophagy-associated proteins. The level of OPTN protein decreased with the E50K mutation in both neuronal types (Fig. 5J, K). Additionally, there was a higher cytosolic LC3-I level in RGCs compared to cortical neurons (Fig. 5J, lane 1 and 3), and only OPTN(E50K) RGCs exhibited a significant increase in the lipidated form of LC3-II (Fig. 5J, L, M). Unlike the observed accumulation of OPTN protein in RGCs (Fig. 1E), OPTN puncta did not deposit in cortical neurons with the E50K mutation (Fig. 5N–Q), while the E50K mutation led to overall protein level reduction after chloroquine treatment (Fig. 5R–W). The abundance of p62 puncta was not affected overall in cortical neurons (Figure S8). Collectively, our findings suggest that the OPTN(E50K) mutation selectively rendered RGCs more vulnerable to neurodegeneration, with data suggesting a greater autophagic demand in RGCs that may be associated with their selective degeneration.
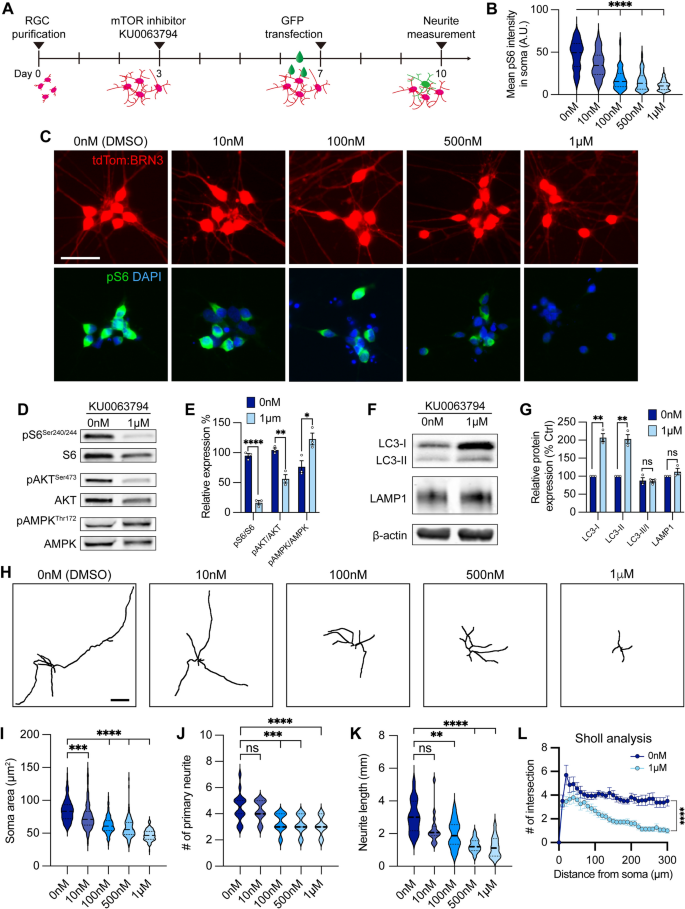
Inhibition of mTOR signaling results in neurodegenerative phenotypes in otherwise healthy hPSC-RGCs
To evaluate whether decreased mTOR activity was associated with impaired RGC neurite outgrowth and autophagy modulation observed in OPTN(E50K) RGCs, we examined how healthy control hPSC-RGCs were affected when treated with the dual mTORC1/2 inhibitor KU0063794 for one week (Fig. 6A). RGCs exhibited a reduction of pS6Ser240/244 intensity following treatment with KU0063794 in a dose-dependent manner (Fig. 6B, C). When treated with KU0063794 (1 µM), RGCs revealed significant decreases in the expression of both mTORC1 and mTORC2 effectors pS6Ser240/244 and pAKTSer473, respectively, while the level of pAMPKThr172 was increased (Fig. 6D, E). Pharmacological inhibition of mTOR also resulted in increased LC3-I and LC3-II, an indication of autophagy activation (Fig. 6F, G). However, the level of autophagic flux (LC3-II/LC3-I) and lysosome protein LAMP1 did not change under mTOR inhibition when compared to vehicle control, suggesting that wild-type RGCs can effectively balance cellular homeostasis between autophagy and acute mTOR inhibition. Analyses of RGC neurites demonstrated a decrease in neurite complexity that correlated with an increase in the concentration of KU0063794 (Fig. 6H–L). As morphological features of RGCs were modulated by KU0063794 in a dose-dependent manner, these results suggest that the pathological hallmarks of neurite retraction observed in OPTN(E50K) RGCs may be the result of progressively decreased mTOR signaling.
mTOR inhibition contributed to neurite shortening in hPSC-derived RGCs. A Schematic timeline of mTOR inhibitor treatment and methods for neurite analyses. B–C Quantification and immunostaining revealed that pS6Ser240/244 intensity was dose-dependent in response to mTOR inhibition in hPSC-RGCs (n = 3 biological replicates using vehicle (DMSO) n = 162, 10 nM n = 143, 100 nM n = 128, 500 nM n = 121, and 1 μM n = 133 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001 for each KU0063794 treatment group compared to vehicle treatment). Scale bar: 25 μm. D–G Western blot and quantification of the relative protein expression displayed a decrease of mTOR signaling and induction of autophagy when mTOR was inhibited by KU0063794 treatment in hPSC-RGCs (n = 3 for each vehicle (DMSO) and 1 μM of KU0063794 treatment, t-test, pS6Ser240/244 ****p < 0.0001, pAKTSer473 **p = 0.0033, pAMPKThr172 **p = 0.0292, LC3-I ***p = 0.0006, LC3-II **p = 0.001, LC3-II/I p = 0.838, LAMP1 p = 0.23). H Representative neurite tracings of vehicle and KU0063794 treatment in hPSC-RGCs. Scale bar: 200 μm. I–L Quantitative analysis of neurite parameters displayed neurite deficits and decreased neurite complexity following mTOR inhibition in hPSC-RGCs as measured by their soma area (n = 3 biological replicates using vehicle n = 107, 10 nM n = 109, 100 nM n = 97, 500 nM n = 107, and 1 μM n = 93 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001) I, number of primary neurites (n = 3 biological replicates using vehicle n = 16, 10 nM n = 16, 100 nM n = 16, 500 nM n = 17, and 1 μM n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, ns = not significant, p > 0.05) J, total neurite length (n = 3 biological replicates using vehicle n = 16, 10 nM n = 16, 100 nM n = 16, 500 nM n = 17, and 1 μM n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, **p < 0.01, ns = not significant, p > 0.05) K, and Sholl analysis (n = 3 biological replicates using vehicle n = 16, and 1 μM n = 15 technical replicates; t-test, ****p < 0.0001) L. Data are all represented as mean values ± SEM
Altered mTOR signaling and autophagy modulated RGC neurodegenerative phenotypes
Insulin is a canonical mTOR activator and previous studies have demonstrated the ability of insulin to rescue neurodegenerative phenotypes in dendritic arbors in rodent RGCs subjected to optic nerve crush [49]. We have demonstrated that OPTN(E50K) RGCs exhibit morphological and functional deficits as soon as 4 weeks after the purification and maturation from retinal organoids (Fig. 5A–C) [12]. However, as insulin is a common component of many cell culture media supplements (such as N2 and B27 supplements), it was present in prior experiments to act upon RGCs and modulate mTOR signaling. Thus, we investigated whether insulin deprivation can lead to a faster disease phenotype in OPTN(E50K) RGCs through a reduced activity of the mTOR signaling pathway. RGCs were grown with medium supplemented with B27 either with or without insulin (normal levels of insulin provided by B27 supplement, or in the complete absence of insulin), and RGC neurites were measured from 1 to 4 weeks of maturation following purification (Figure S9 A-C). As soon as 2 weeks following purification, OPTN(E50K) RGCs subjected to insulin deprivation exhibited neurite deficits, while OPTN(E50K) RGCs with insulin revealed robust neurite outgrowth comparable to wild-type RGCs with or without insulin (Figure S9 D-G), as measured by soma area, neurite length, number of primary neurites and Sholl analysis (Figure S9 H-K). Insulin deprivation also decreased the mTORC1 effector pS6Ser240/244 in OPTN(E50K) RGCs (Figure S9 L-N), as well as significantly increased the level of LC3-II (Figure S9 O-Q), suggesting an imbalance of autophagy and mTORC1 in OPTN(E50K) RGCs when deprived of insulin, resulting in RGC neurite morphological deficits. Moreover, as insulin mediates glucose metabolism [59], we next determined whether the absence of insulin in the culture media would induce metabolic stress in RGCs. Our results show that RGCs subjected to insulin deprivation exhibited reduced glucose uptake (Figure S10), suggesting increased metabolic stress induced by the absence of insulin. These results supported the idea that insulin signaling is essential to promote overall RGC neurite outgrowth, and that a lack of sufficient mTOR signaling results in neurite retraction and deficient glucose uptake.
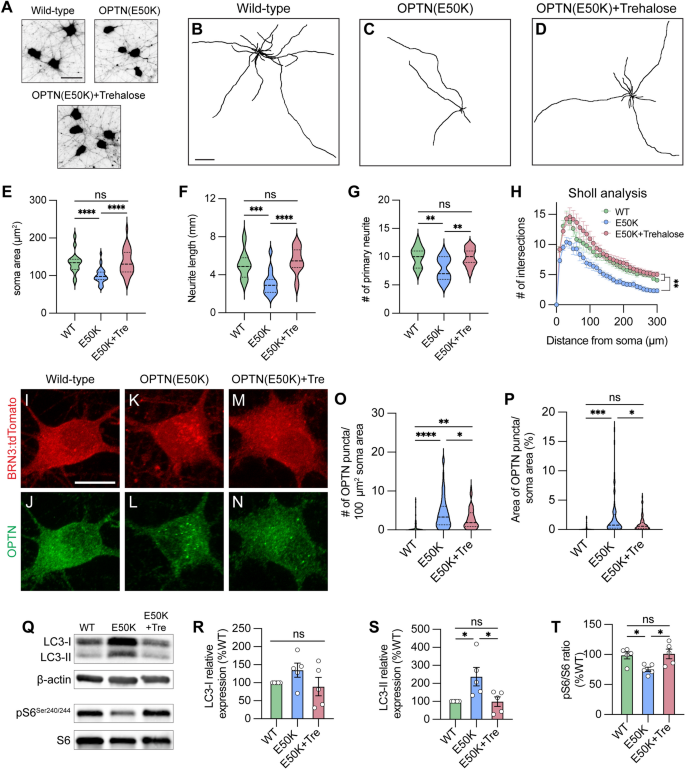
We have previously identified that autophagy deficits and the accumulation of autophagosomes can be cleared after a short term (24 h) treatment with the autophagy inducer rapamycin in OPTN(E50K) retinal organoids [12]. However, as rapamycin induces autophagy via mTOR inhibition, long term treatment with rapamycin would not represent an ideal approach due to the resulting decrease in mTOR signaling. As RGC survival relies upon the homeostatic balance between mTOR and autophagy signaling [60], we hypothesized that inducing autophagy in a manner that may also preserve mTOR signaling might rescue neurodegenerative phenotypes in OPTN(E50K) RGCs. To accomplish this, we used the compound trehalose to stimulate the autophagic-lysosomal degradation pathway in RGCs, which has been extensively characterized to function as an autophagy activator in an mTOR-independent manner [61], and has also been shown to reduce protein aggregates in a number of other neurodegenerative diseases [62,63,64,65,66]. In our experiments, OPTN(E50K) RGCs demonstrated a robust protection of neurite morphology, measured by a preservation of soma area, neurite length, number of primary neurites, and Sholl analysis following 2 weeks of trehalose treatment when compared with wild-type RGCs as well as untreated OPTN(E50K) RGCs (Fig. 7A–H). Additionally, trehalose treatment partially reduced OPTN puncta accumulation in OPTN(E50K) RGCs (Fig. 7I–P). Moreover, trehalose treatment reduced LC3-II expression as well as sustained levels of the mTORC1 effector pS6Ser240/244 (Fig. 7Q–T), collectively suggesting that treatment with trehalose can at least partially restore autophagic balance, while at the same time maintaining mTOR signaling, respectively, leading to sustained overall health of OPTN(E50K) RGCs.
mTOR-independent activator prevents neurite retraction and clears protein accumulation in OPTN(E50K) hPSC-RGCs. A Representative images of soma area in wild-type, OPTN(E50K), and trehalose-treated OPTN(E50K) hPSC-RGCs. Scale bar 25 μm. B–D Representative neurite tracing images of wild-type, OPTN(E50K), and trehalose-treated OPTN(E50K) hPSC-RGCs. Scale bar: 200 μm. E–H Quantitative analysis of neurite parameters demonstrated protection of neurites in OPTN(E50K) hPSC-RGCs after trehalose treatment for 2 weeks, as measured by the soma area (n = 3 biological replicates using WT n = 30, E50K n = 30, and E50K-trehalose n = 30 technical replicates, One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ns = not significant, p > 0.05) E, number of primary neurites (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, ns = not significant, p > 0.05) F, total neurite length (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. **p < 0.01, ns = not significant, p > 0.05) G, and Sholl analysis (n = 3 biological replicates using WT n = 15, E50K n = 15, and E50K-trehalose n = 15 technical replicates; One-way ANOVA, Tukey post hoc test. WT vs E50K: **p = 0.0095; WT vs E50K(trehalose): ns = not significant, p = 0.81; E50K vs E50K(trehalose): **p = 0.0012) H. I–N Immunostaining displayed the decrease of OPTN puncta in OPTN(E50K) hPSC-RGCs after trehalose treatment. Scale bar: 10 μm. O, P Quantification of OPTN puncta in hPSC-RGCs (n = 3 biological replicates using WT n = 51, E50K n = 60, and E50K-trehalose n = 61 technical replicates; One-way ANOVA, Tukey post hoc test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns = not significant, p > 0.05). Q–T Western blot and the relative protein expression demonstrated the recovery of changes to LC3-II and pS6Ser240/244 expression in OPTN(E50K) hPSC-RGCs after trehalose treatment. (n = 5 for each WT, E50K, and E50K with trehalose treatment; One-way ANOVA, Tukey post hoc test. LC3-I: WT vs E50K: p = 0.41, WT vs E50K(trehalose): p = 0.91, E50K vs E50K(trehalose): p = 0.23. LC3-II: WT vs E50K: p = 0.03, WT vs E50K(trehalose): p = 0.99, E50K vs E50K(trehalose): p = 0.03. pS6: WT vs E50K: p = 0.048, WT vs E50K(trehalose): p = 0.95, E50K vs E50K(trehalose): p = 0.029.). Data are all represented as mean values ± SEM





Add Comment