Between December 19, 2006, and December 17, 2022, the subjective physical activity measurement dataset recorded 5562 deaths among 105,705 individuals during a median follow-up of 13.47 years (IQR 12.89–14.20; 1417869 person-years), which were included in the lifestyle-associated analysis. The objective physical activity measurement dataset comprised 42,006 participants, with 1580 deaths recorded over a median follow-up period of 13.6 years (IQR 12.98–14.29; 571591.9 person-years). Baseline characteristics of the study population are displayed in Table 2.
Optimal life patterns for delaying biological ageing
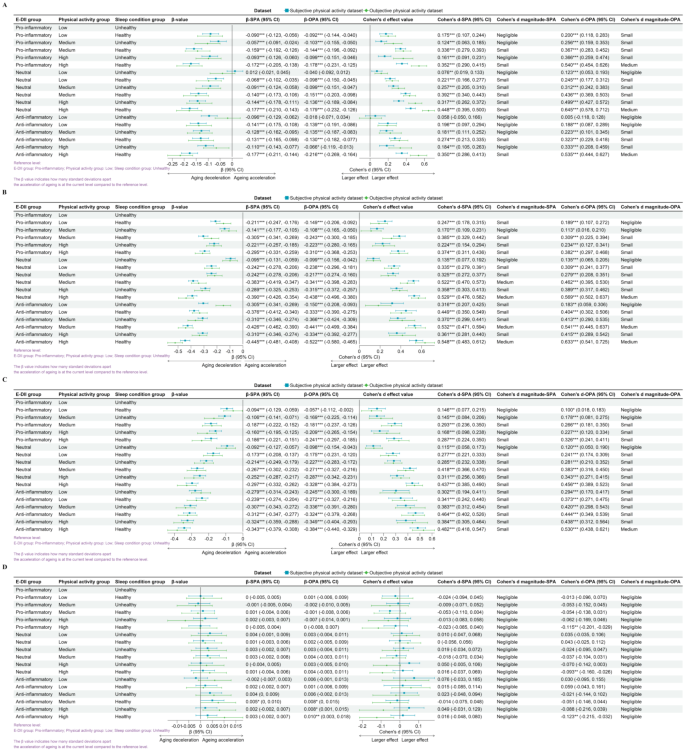
From Fig. 1, it is evident that compared to the reference level (least favorable lifestyle pattern: pro-inflammatory diet, low physical activity, and unhealthy sleep conditions), any improvement in the dietary inflammation index, physical activity, or sleep conditions is associated with lower biological age (Supplementary Material: Figure S3.3 and Figure S3.4). However, these effects are mostly below or equal a moderate effect size.
The relationship between lifestyle patterns and the acceleration of biological aging. The figure includes homeostatic dysregulation (A), KDM biological age acceleration (B), phenotypic age acceleration (C), and leucocyte telomere length (D). Reference level: proinflammatory in the E-DII group; low in the physical activity group; unhealthy in the sleep condition group. The β-value indicates how many standard deviations of biological age-accelerated change in that level compared to the reference level. Cohen’s d indicates the estimated effect size
Abbreviations: SPA: subjective physical activity measurement data set; OPA: objective physical activity measurement data set; E-DII: energy-adjusted dietary inflammation index.
In the study examining the impact of lifestyle patterns on HD, we observed similar effects across SPA and OPA. The strongest association was observed in the group with a neutral diet, high physical activity level, and healthy sleep conditions, with Cohen’s d for SPA at 0.448 (95% CI: 0.395, 0.500) and for OPA at 0.645 (95% CI: 0.578, 0.712). In the weighted linear model, after adjusting for covariates, the strongest association was observed in the group with an anti-inflammatory diet, high physical activity level, and healthy sleep conditions, with β for SPA at -0.177 (95% CI: -0.211, -0.144) and for OPA at -0.216 (95% CI: -0.269, -0.164).
Regarding the influence of lifestyle patterns on KDM biological age acceleration, similar effects were found across SPA and OPA. The strongest association was present in the group with an anti-inflammatory diet, high physical activity level, and healthy sleep conditions, with Cohen’s d for SPA at 0.548 (95% CI: 0.483, 0.612) and for OPA at 0.633 (95% CI: 0.541, 0.725). Even after adjusting for covariates, this group maintained the strongest association, with β for SPA at -0.445 (95% CI: -0.481, -0.408) and for OPA at -0.522 (95% CI: -0.580, -0.465).
In the study on the impact of lifestyle patterns on phenotypic age acceleration, we found similar effects across SPA and OPA. The strongest association was again observed in the group with an anti-inflammatory diet, high physical activity level, and healthy sleep conditions, with Cohen’s d for SPA at 0.482 (95% CI: 0.418, 0.547) and for OPA at 0.530 (95% CI: 0.438, 0.621). After covariate adjustment, the strongest association persisted in this group, with β for SPA at -0.343 (95% CI: -0.379, -0.308) and for OPA at -0.384 (95% CI: -0.440, -0.329).
In the research on the effects of lifestyle patterns on telomere length, we noted a divergence in the effects between SPA and OPA. In the SPA, a significant association was only observed after covariate adjustment in the group with an anti-inflammatory diet, moderate physical activity level, and healthy sleep conditions, with β for SPA at 0.005 (95% CI: 0, 0.01). Conversely, in the OPA, the strongest association was found in the group with an anti-inflammatory diet, high physical activity level, and healthy sleep conditions, with Cohen’s d for OPA at -0.123 (95% CI: -0.215, -0.032), and the largest effect persisted after covariate adjustment, with β for OPA at 0.01 (95% CI: 0.003, 0.018).
The association between lifestyle patterns and biological age acceleration exhibits significant differences between gender groups only within certain lifestyle groups, with most groups showing no gender-specific variations (Supplementary Material: Figure S3.5 and Figure S3.6). In different age cohorts, individuals under 60 years of age showed stronger associations between lifestyle patterns and HD compared to those over 60. Conversely, the associations between lifestyle patterns and KDM biological aging and phenotypic aging was more pronounced in the population over 60 than in those under 60 (Supplementary Material: Figure S3.7 and Figure S3.8). Within various chronic disease status groups, individuals with a single chronic condition exhibited stronger associations between lifestyle pattern and HD than other groups; those with multimorbidity showed stronger associations with phenotypic age acceleration.
In summary, our findings suggest that maintaining an anti-inflammatory diet, at least moderate level physical activity, and healthy sleep conditions are associated with a significant small to medium level difference in physiological aging markers in most scenarios.
Biological age aging and all-cause mortality
An elevation of one standard deviation in KDM biological age acceleration is associated with a 30.8% higher all-cause mortality risk (Table 3, HR-SPA = 1.308, 95% CI: 1.273, 1.419), and a similar pattern is observed with a 29.6% increase (HR-OPA = 1.296, 95% CI: 1.232, 1.364). Relative to the reference level (|KDM-age acceleration| ≤ 1), extreme deviations in KDM biological age acceleration (< -1 or > 1) are linked to HR-SPA values of 0.871 (95% CI: 0.802, 0.946) and 1.334 (95% CI: 1.229, 1.447), and HR-OPA values of 0.748 (95% CI: 0.646, 0.866) and 1.213 (95% CI: 1.048, 1.404), respectively.
Similarly, a one standard deviation increase in phenotypic age acceleration is associated with a 33.1% heightened risk of mortality (HR-SPA = 1.331, 95% CI: 1.299, 1.364), and a 28.3% increase (HR-OPA = 1.283, 95% CI: 1.225, 1.344). Against the reference level (|Phenoage acceleration| ≤ 1), participants with Phenoage acceleration < -1 and > 1 exhibit HR-SPA values of 0.867 (95% CI: 0.799, 0.940) and 1.359 (95% CI: 1.256, 1.469), and HR-OPA values of 0.807 (95% CI: 0.696, 0.937) and 1.238 (95% CI: 1.071, 1.430), respectively.
An increase of one standard deviation in telomere length is associated with a 5.4% lower mortality risk (HR-SPA = 0.946, 95% CI: 0.919, 0.972) and a 9.5% decrease (HR-OPA = 0.905, 95% CI: 0.859, 0.953). Compared to the reference level (|Telomere length| ≤ 1), significant telomere length variations (< -1 or > 1) correspond to HR-SPA values of 1.251 (95% CI: 1.168, 1.340) and 0.861 (95% CI: 0.790, 0.939), and HR-OPA values of 1.226 (95% CI: 1.072, 1.402) and 0.837 (95% CI: 0.716, 0.980), respectively.
Kaplan-Meier survival curves substantiate that diminished biological age acceleration or elongated telomere lengths are associated with a reduced all-cause mortality risk (Supplementary Material: Figures S3.11 and S3.12). A non-linear relationship is evident between biological aging acceleration, telomere length, and mortality risk (Supplementary Material: Figure S3.13).
In sum, biological age acceleration and telomere length exhibit significant positive and negative correlations with all-cause mortality. Within the UK Biobank cohort, biological age acceleration emerges as a potent predictor of mortality.
Optimal lifestyle for reducing all-cause mortality
Figure 2 demonstrates that the lifestyle pattern combining an anti-inflammatory diet with moderate physical activity and healthy sleep is associated with the lowest mortality risk (HR-SPA: 0.690, 95% CI: 0.538, 0.884; HR-OPA: 0.493, 95% CI: 0.293, 0.828).
The relationship between lifestyle and all-cause mortality. Reference level: proinflammatory in the E-DII group; low in the physical activity group; unhealthy in the sleep condition group. *P < 0.05, **P < 0.01, ***P < 0.001
Abbreviations: SPA: subjective physical activity measurement data set; OPA: objective physical activity measurement data set; E-DII: energy-adjusted dietary inflammation index.
The pro-inflammatory diet group was associated with higher mortality risk compared to those consuming neutral or anti-inflammatory diets. This pattern was mirrored in the associations between physical activity levels, sleep quality, and mortality risk, with higher risks observed in the low physical activity and unhealthy sleep cohorts (Supplementary Material: Figures S3.14 and S3.15, Table S3.1). Additionally, there were linear or non-linear relationships between E-DII, physical activity level, sleep quality score, and the risk of all-cause mortality (Supplementary Material: Figure S3.16).
Disparities in lifestyle associations on mortality risk were evident between genders. While both sexes showed lower mortality risk with moderate physical activity and healthy sleep, women showed a more pronounced association between lower mortality risk and the combination of an anti-inflammatory diet with moderate activity (Supplementary Material: Figures S3.17 – S3.18).
For individuals under 60, a high-activity, healthy-sleep, anti-inflammatory diet lifestyle was associated with significantly lower mortality risk. In contrast, for those aged 60 and above, the association between these lifestyle factors and mortality risk was less pronounced (Supplementary Material: Figures S3.19 – S3.20).
In the absence of chronic diseases, high levels of physical activity and healthy sleep conditions were significantly associated with a lower risk of all-cause mortality (HR approximately 0.769–0.944). For individuals with chronic conditions, an anti-inflammatory diet, particularly when paired with moderate physical activity, was associated with additional lower mortality risk (HR approximately 0.781–0.909). Those with multiple diseases showed lower mortality risk associated with an anti-inflammatory diet across all physical activity levels, especially moderate (HR-SPA = 0.724, 95% CI: 0.547, 0.96) (Supplementary Material: Figures S3.21 – S3.22).




Add Comment