Study design and procedure
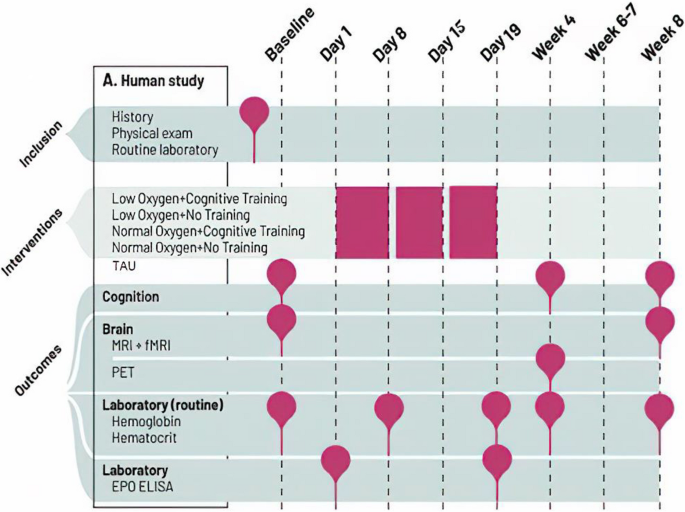
In a basic scientific study in healthy humans, we will determine the causal mechanisms of changes in neuroplasticity and cognition across multiple levels of analysis (sub-study 1). The clinical study (sub-study 2) will in parallel clarify whether targeting these mechanisms translates into cognitive benefits in cognitively impaired patients, in line with a phase II study design. In this way, both studies will provide proof-of-concept evidence for potential benefits of intermittent hypoxia combined with cognitive training on brain plasticity and cognitive functioning. Figure 1 depicts an overview of study events.
Sub-study 1 involves four intervention groups: (1) normobaric hypoxia (12% O2) combined with cognitive training, (2) hypoxia (12% O2) with no training, (3) cognitive training under normoxia (20% O2), and (4) normoxia (20%) with no training in a double-blinded controlled design. This design enables us to disentangle combined and separate effects of altitude-like hypoxia and cognitive training. Participants are randomized in blocks and undergo interventions in these groups for practical reasons. During the three-week treatment, participants breathe 12% ambient oxygen (≈ 4400 m altitude) or approximately normal sea-level oxygen (20%) in a treatment room, 3.5 h daily, six days per week (18 sessions in total). After a blood test (days 1, 6, 20) and light standardized breakfast, they enter the treatment room. Here, they sit at a desk separated by desk dividers. On iPads, they perform cognitive training or matched control games without cognitive benefits. Cognitive training is interleaved by short breaks, during which the participants can relax or walk on a treadmill inside the room. This is to avoid complete physical inactivity throughout the 3.5 h sessions and thereby make the sessions more pleasant for participants. Participants undergo cognition assessments in weeks 1 (baseline), 4, and 8 and functional and structural MRI in weeks 1 and 8, when red blood cell counts are comparable between groups. Participants in the two extreme groups (normobaric hypoxia with cognitive training and normoxia with no training) will also undergo PET scanning in week 4 (post-treatment). Participants will be recruited consecutively for the PET scans until an equal distribution in each of the extreme intervention arms (i.e., hypoxia with cognitive training and normoxia with no training), sex, and age group is achieved.
Sub-study 2 involves two intervention groups: (1) normobaric hypoxia (12%) combined with cognitive training 3.5 h daily, five to six days per week for three weeks (16 sessions in total), and (2) treatment as usual (TAU) in an assessor-blind controlled design. Patients in the TAU group will initially be assessed at the same time points as the active group with neurocognitive testing at baseline, week 4, and week 8, fMRI at baseline and week 8, and PET in week 4. After completed testing at week 8, patients in the TAU group undergo the three-week active intervention, followed by one additional neurocognitive assessment and mood rating in the week after treatment completion. The inclusion of this TAU arm rather than an active control group was chosen to ensure patient motivation and minimize attrition in this time-intensive study. Similar study designs with TAU control groups are common in pro-cognitive intervention studies investigating novel treatments in mood disorders and schizophrenia [39, 40]. Although patients will not be blinded to their allocated treatment arm, neurocognitive test results are generally not susceptible to placebo effects [41, 42] and assessments will be carried out by blinded, fully trained research assistants (psychology students).
Participants
For sub-study 1, we will recruit 120 healthy participants with no current or prior psychiatric illness. For sub-study 2, we will recruit 60 patients with affective disorders (MDD or BD) in partial or full remission (Hamilton Depression Rating Scale 17-items (HDRS-17; [43]) and the Young Mania Rating Scale (YMRS; [44]) score ≤ 14). Healthy participants are primarily recruited across the University of Copenhagen and other higher education facilities in Copenhagen to ensure that participants are able to take time off for the time-intensive study. Patients are mainly recruited through the Mental Health Services, Capital Region of Denmark, and through advertisements on relevant websites.
For sub-study 1, eligible participants are between 18 and 50 years of age, be fluent in Danish, and have no psychiatric history. For sub-study 2, patients are between age 18 and 65, be fluent in Danish, have a diagnosis of BD or MDD confirmed using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) [45], in partial or full remission (defined as a score of ≤ 14 on HDRS-17 and YMRS with objectively verified cognitive impairment according to Screen for Cognitive Impairment in Psychiatry (SCIP) [46] and/or self-reported cognitive impairment measured with Cognitive Complaints in Bipolar disorder Rating Assessment (COBRA) [47]. For SCIP, their performance must be > 0.5 standard deviations (SD) below their demographically adjusted expected total SCIP score or on minimum two SCIP subtest scores [48]. For COBRA, patients must report substantial cognitive impairment defined as a score ≥ 14 [47, 49]. We include a subjective measure of cognitive impairment as eligible patients can have suffered a cognitive decline from a high level of premorbid functioning, which may not be detectable by a cross-sectional evaluation with SCIP.
Common exclusion criteria for both sub-studies are schizophrenia or schizoaffective disorder, organic mental disorders (ICD-10 codes F00-09), history of neurological disorder (including dementia), alcohol or substance abuse, daily use of ≥ 22.5 mg oxazepam, or history of serious head trauma. To ensure safety, candidates are also excluded if they have previous altitude sickness, significant medical conditions (e.g., heart disease, diabetes, renal failure, untreated/insufficiently treated hypertension, and/or thrombosis), history of epilepsy or thromboembolic events, first-degree family with thromboembolic events before age 60, are pregnant or breastfeeding, currently take iron supplements, smoke, use other nicotine products regularly, or have a BMI > 30. All female participants who are not using hormonal contraceptives must take a pregnancy test before beginning the intervention to ensure that they are not pregnant. Participants are also excluded if they have received electroconvulsive therapy (ECT) three months prior to participation or are dyslexic. Regarding fMRI assessments, participants are not eligible if they suffer from claustrophobia and have a pacemaker and/or other metal implants inside their bodies. Participants are not eligible for PET assessments if they have participated in experiments with radioactivity (> 10 mSv) within the last year, have significant occupational exposure to radioactivity, or if they take medication incompatible with study aims (e.g., SV2A binding agents). Patients with mood disorders, who do not meet the fMRI and PET inclusion criteria, will not be excluded from the trial per se, but only from these neuroimaging assessments. All participants must provide written informed consent. The SPIRIT reporting guidelines were used for the current study [50]. See Appendix A for a completed SPIRIT checklist.
Study setting
The ALTIBRAIN trial, including all neurocognitive assessments and intervention sessions, will be conducted at the NEAD (Neurocognition and Emotion in Affective Disorders) Centre, Department of Psychology, University of Copenhagen, and Psychiatric Center Copenhagen, Frederiksberg Hospital, Denmark. Furthermore, MR and PET scans are conducted at the Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Randomization and blinding
Randomization is conducted in the database program REDCap [51], which is also used to store the collected data, utilizing a 1:1 allocation ratio. Randomization occurs after inclusion of the participants planned to undergo treatment sessions together. Treatment groups are stratified for age (> 27 in sub-study 1 of generally young volunteers/ > 35 in sub-study 2 of patients). For randomization purposes, date of birth is registered to determine the stratum to which the participant belongs at the time of enrolment. Study identification numbers are given consecutively within each stratum and treatment arm. Randomization is conducted in the randomization module in REDCap, where an un-blinded researcher inputs the stratum and the program then retrieves the allocated intervention. To ensure blinding of the outcome-assessors, the randomization module in REDCap is only accessible to the researchers, who are not involved in evaluation of the efficacy parameters.
Healthy participants in sub-study 1 are blind to the oxygen condition since the low/normal oxygen air blown into the room is of equal temperature and humidity. Furthermore, they are told that they receive one of two forms of cognitive training, which effectively blinded participants in a previous study [52]. After study completion, we will assess whether blinding has been maintained for each participant, since there is a small risk that some participants develop symptoms of mild altitude sickness which could unblind them to their intervention. Blinding of the outcome assessors is ensured by their absence during the training visits (where oxygen levels are displayed and monitored on a screen outside the room). Participants will be instructed not to disclose any information concerning their intervention during outcome assessments and under no circumstances will the allocation be revealed to the outcome assessors. The training visits are conducted by researchers who are not involved in outcome assessments.
Intervention: hypoxia cognition training
Low/normal ambient oxygen
Fresh air with 12% or 20% O2 is blown into a sealed 20 m3 room by a 4 kW air compressor with a safety-approved system developed by HöhenBalance, Austria (see Fig. 2 for experimental setup). For the active condition, participants enter the room at 16% O2 (≈ 2,200 m altitude). Upon entry, the O2 levels will be reduced from 16 to 12% (≈ 4400 m altitude) in a 30-min lead-in phase. The precise control by the HöhenBalance systems allows control of O2 levels to ± 0.1%. The target O2 level of 12% will be maintained over three hours.
HöhenBalance systems create simulated altitude by introducing normobaric breathable hypoxic air. Therefore, even under any conceivable worst-case failure condition, the systems cannot give rise to any potentially harmful oxygen condition to persons inside or outside the room. A password access control provides additional safety.
Cognitive training and matched computer games
The web-based cognitive training (Happy Neuron Pro) is grounded on principles of neuroplasticity-based learning by being intensive, neuroadaptive, engaging, and rewarding. This web-based training program has been translated into Danish and is used for traumatic brain injury, schizophrenia, and in our previous cognitive remediation trial ([53] and www.happyneuronpro.com), making its implementation feasible. Participants in the no training control condition receive computer games similar to Happy Neuron Pro but with low cognitive demand that produce no cognitive benefits [52]. Specifically, this sham procedure involves the exact same stimuli as the active condition but with changes from trial to trial only in the appearance of the tasks. In contrast, the active training involves parametric task adjustment by decreasing stimuli presentation time, increasing working memory load, decreasing time to respond, and increasing the number of non-target items (distractors).
Outcome measures
For an overview of outcome assessment domains, measures, frequency, and timing, see Table 1. The outcome measures listed below are consistent with the latest recommendations from the International Society for Bipolar Disorders (ISBD) Task Force [54].
Primary outcome measure
The primary outcome measure is changes in a cognitive composite score, consisting of neuropsychological tests covering attention, memory, and executive functions from baseline to week 4. We have previously demonstrated an improvement on this speed of complex cognitive processing composite measure in patients with BD after 8 weeks of EPO treatment [28]. In the present trial, the specific tests included in the primary composite outcome measure are the Rey Auditory Verbal Learning Test (RAVLT) [55], The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Coding [56], Verbal Fluency with the letter “D” [57], Wechsler Adult Intelligence Scale (WAIS)-III Letter-Number Sequencing [58], Trail Making Test Part B (TMT-B) [59], and Rapid Visual Information Processing (RVP) from the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition Ltd.). To derive the cognitive composite score, we will z-transform and sum performance scores from RAVLT total recall, TMT-B, WAIS-III Letter-Number Sequencing, RBANS Coding, Verbal Fluency (letter “D”), and RVP speed for correct responses [28].
Secondary outcome measures
The secondary cognitive outcome measure consists of mean choices to correct in the One Touch Stockings of Cambridge (OTS) test from CANTAB, which yielded particularly strong effects of cognitive training with Happy Neuron in our previous study [60]. Dorsal prefrontal cortex (dPFC) activity during spatial working memory (N-back task) is another secondary outcome measure because the dPFC is consistently engaged in both healthy participants and patients with mood disorders in response to successful pro-cognitive interventions [61].
Additionally, sub-study 2 of patients with mood disorder includes a measure of daily functioning as secondary outcome in accordance with the ISBD recommendations [54]. The effect of the intervention on daily functioning is assessed with a novel virtual reality test of daily life cognitive skills, the Cognition Assessment in Virtual Reality (CAVIR) test [62]. The CAVIR test is an engaging, immersive, and self-administered 360° VR test in a kitchen, where the participant’s cognitive skills related to planning and preparing a meal are assessed, thus enabling insight into patients’ daily life cognitive skills.
Tertiary outcome measures
The tertiary cognitive outcome measures are the RAVLT, RBANS Coding and Digit Span, Verbal Fluency with the letters “S” and “D”, WAIS-III Letter-Number Sequencing, the Wisconsin Card Sorting Task (WCST), the Rapid Visual Information Processing (RVP; CANTAB), additional measures from the One Touch Stockings of Cambridge (OTS; CANTAB), the spatial working memory (SWM; CANTAB), and the Emotion Recognition Test (ERT; CANTAB), as well as the Trail Making Test Part A (TMT-A) and B (TMT-B), respectively.
The Assessment of Quality of Life (AQoL) [63], the Cognitive Complaints in Bipolar Disorder Rating Assessment (COBRA) [47], Sheehan Disability Scale (SDS) [64], the World Health Organization Quality of Life (WHOQOL-BREF) [65], the Work and Social Adjustment Scale (WSAS) [66], and the Pittsburgh Sleep Quality Index (PSQI) [67] will be applied. Finally, psychosocial functioning is also assessed with the clinician-rated interview Functional Assessment Short Test (FAST) [68]. History of early life stress will be assessed with the Childhood Abuse and Trauma Scale [69] at the time of inclusion.
To minimize learning effects on neuropsychological test with repeated testing at the follow-up assessments, alternate versions of the RAVLT (original list AB, GeAB, and Cr-AB) and RBANS Coding and Digit Span (version A and B) are used. These versions are administered in counter-balanced order within each stratum. Finally, premorbid verbal IQ is assessed with the Danish Adult Reading Test [70].
Additional mechanistic outcome measures
Cognition-related neural activity will be assessed with resting state fMRI and the following carefully selected fMRI tasks: (i) a spatial N-back working memory task and (ii) a novel grocery list strategic encoding paradigm. Alternate matched versions of the grocery list fMRI paradigm are used at the two testing times to minimize neural desensitization to the test stimuli. Structural MRI measures of interest are hippocampal and cortical volume, cortical thickness, and white matter integrity. The PET measure of interest is hippocampal and prefrontal cortex SV2A [11C]UCB-J binding as a readout of presynaptic density, as stated in the aims.
To further increase insight into the underlying neurobiological mechanisms involved in the potential beneficial cognitive effects of hypoxia cognition training, blood samples from first day (day 1) and second to last day of treatment (day 19) will be analyzed for the exploratory purpose of investigating whether baseline levels and/or changes of peripheral biomarkers, including EPO, are correlated with cognitive improvement.
Blood based biomarkers of neuroplasticity (e.g., brain derived neurotrophic factor), inflammation (e.g., interleukin factors), and EPO will be obtained at baseline (BL) and end of treatment for explorative analyses.
Biochemistry
Blood test data is pseudo-anonymized by the Clinical Biochemistry Department—Section for External Projects, Copenhagen University Hospital. This is done by producing serial numbers that are attached to the individual blood tests and a separate electronic conversion key linking the serial numbers to participants ID numbers. Research blood samples will be transferred to the Neuropsychiatric Laboratory, Frederiksberg Hospital, and stored at − 80 °C until use. The collected blood samples will be stored until they are analyzed in a research biobank set up for this project. Measurements will be performed at Department of Clinical Pharmacology, Rigshospitalet. The research biobank will cease at the end of the project (expected date 01 January 2029), and any excess material will then be transferred to Cimbi Biobanken, which is an existing and approved biobank for future research under the Neurobiological Research Unit, Copenhagen University Hospital, Rigshospitalet.
Statistical analyses
Primary, secondary, tertiary, and exploratory outcome measure analyses
Behavioral data from neuropsychological test score performance, subjective cognitive impairments, quality of life, level of functioning, psychosocial functioning, and mood symptoms (i.e., data from the primary, secondary, and tertiary outcomes) will be analyzed using mixed model design. Hence, the analyses investigate how the mean score of each outcome changes over time in each treatment group and testes for interaction effects (i.e., are the trajectories different between the groups). All analyses will be based on an intention-to-treat (ITT) principle. Hence, all participants will be analyzed based on their allocated intervention arm. Participants with incomplete data will also be included in the analyses without any ad hoc imputation [71]. No interim analyses will be performed. A nonblinded researcher will pseudo-anonymize the data prior to analysis, and data analyses will thus be conducted by blinded researchers.
MRI analyses
Whole-brain and hippocampal volume and cortical thickness will be accessed using the FMRIB Software Library (FSL) vertex-based analysis and FreeSurfer Image Analysis Suite, respectively. White matter integrity is assessed with tract-based spatial statistics.
Resting state and task-based functional MRI (fMRI) data will be preprocessed and analyzed with the FMRIB Expert Analysis Tool (FEAT; latest version available at trial completion) part of FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl). For resting-state fMRI data, we will use Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) to conduct group-level ICA by multi-session temporal concatenation, identifying the common resting state networks across the groups. Furthermore, fMRI data from the N-back working memory task and the grocery list encoding and retrieval tasks will be analyzed using a region-of-interest analysis to assess differences between the intervention and control groups in neural activity in the dorsal PFC (dPFC) after completing 4 weeks of treatment (adjusting for any difference in neural activity at baseline). We will also conduct volume-of-interest analyses of the dPFC for the N-back task and for both the dPFC and hippocampi for the word encoding task to investigate our hypotheses. Finally, exploratory whole-brain analyses will be conducted to investigate any treatment-related effects in other brain regions.
Positron emission tomography analyses
Dynamic [11C]UCBJ-J PET imaging will be acquired over 90 min using the High Resolution Research Tomograph (HRRT; CTI/Siemens, Knoxville, TX, USA) PET scanner as previously described [72]. Following motion-correction, the PET data are processed using the PVElab software pipeline (https://nru.dk/index.php/allcategories/category/30-software), or similar, where PET images are co-registered to participants’ T1-weighted 3 T MR image, and regions of interest are automatically delineated [73].
Radioactivity concentrations are extracted from grey matter to generate regional time-activity curves (TACs) and subsequently used for kinetic modeling. Quantification is performed using the simplified reference tissue model 2 (SRTM2) with the white matter region centrum semiovale as pseudo-reference tissue. The outcome is the non-displaceable binding potential (BPND; ratio of specifically bound radioligand to that of non-displaceable radioligand in tissue).
Sample size and power calculation
Power calculations were performed with the software program G*Power 3.1.9.4 [74] and were based on the primary hypothesis that altitude-like hypoxia combined with cognitive training produces robust sustained cognitive improvement compared with normoxia and no training (hypothesis i). As hypoxia triggers the endogenous production of EPO, we based the power calculation on our previous clinical study of subcutaneous EPO treatment in which we demonstrated clinically relevant cognitive enhancement [75]. In that study, the differential change between EPO and placebo groups in the same cognitive composite score as we apply in the present study was 0.5 standard deviations (SD) [75]. Assuming an effect of 0.4 SD differential change (medium effect size) between the two groups and 0.5 SD of the change, we will achieve > 80% power to detect an effect at an alpha level of 0.05 by including 26 participants per group. This number of participants provides adequate power for the structural and functional MRI investigations based on our previous studies of EPO [30, 32, 33]. To accommodate for up to a 20% drop-out, we will include 30 participants per treatment arm; i.e., 120 participants in sub-study 1 and 60 patients in sub-study 2.
For PET imaging of SV2A [11C]UCB-J binding (reflecting presynaptic density), power calculations involved the following assumptions: (i) the average (SD) BPND in the frontal cortex in healthy participants is 3.36 (0.38) (based on in-house data), and (ii) the combined intervention will lead to a 10% increase in BPND. With these assumptions, we will achieve a power of 0.82 to detect an effect at significance level of 0.05 post-treatment of the combined intervention (primary aim) with n = 20 per treatment arm using a two-tailed, independent-sample t-test.
Data management and monitoring
All personal information will be obtained at the eligibility assessment or from patient records in cases where participants are unable to provide the needed information. Pseudo-anonymized data from the neuropsychological tests, virtual reality test, questionnaires, interviews, and functional assessment will be registered in the REDCap database, which meets requirements from the Danish Data Protection Agency. Pseudo-anonymized raw paper data from neuropsychological tests are kept in a locked filing cabinet. Questionnaires and interviews are collected directly in REDCap. Data quality is ensured by score range restrictions on values for all outcomes, and all data for the primary and secondary outcome measures will be doublechecked prior to analyses. Signed consent forms as well as a list that matches participant ID numbers with personal information are kept separate from pseudo-anonymized data. The key matching participants’ personal information with their ID number will be deleted and consent forms maculated 10 years after study completion. At this point, all data will be completely anonymized. All trial authors will have access to the final trial dataset. If a participant is excluded or withdraws from the study, the reason for exclusion is documented in REDCap, along with information regarding any adverse events.
Participant retention
In accordance with the Danish National Scientific Ethics Committee’s guidelines for remuneration or other benefits to voluntary participants, all healthy participants (sub-study 1) will receive remuneration corresponding to their time participating in neuropsychological examinations, neuroimaging, and treatment sessions. Patients (sub-study 2) will be offered feedback on the results of their neuropsychological assessments after completing the final assessment. In accordance with the Danish National Scientific Ethics Committee’s guidelines for remuneration or other benefits for patient participants, they will receive gift cards for neuropsychological examinations and neuroimaging. In addition, all participants in both studies will receive transport allowance in connection with the various study days.




Add Comment