A. muciniphila is drastically depleted from the fecal microbiome of human subjects with diseases associated with cognitive dysfunction
The study included 97 patients diagnosed with liver cirrhosis (without HE, n = 58; with HE, n = 39) and 57 healthy controls. In addition, 48 cognitively impaired subjects (Mini-Mental State Examination [MMSE]-KC score ≤ 24) and 32 healthy controls (MMSE-KC score ≥ 25) classified by an examination of the MMSE score were included. Table 1 and Table S1 present the detailed characteristics of each group, including clinical, metabolic, and biochemical profiles. Cirrhotic patients with HE had lower levels of ALT (P = 0.0009) (Supplementary Fig. 1A), AST (P < 0.0001) (Supplementary Fig. 1A), gamma-glutamyl transferase (P < 0.0001), total cholesterol (P < 0.0001), and triglycerides (P < 0.0001) than cirrhotic patients without HE. No differences in blood biochemical profiles were observed between the test groups classified using the MMSE.
We analyzed 16S rRNA gene sequencing data to compare the distribution of microbe proportions according to the presence or absence of disease. The proportions of dominant taxa at the phylum (Fig. 1A) and family levels are shown (Supplementary Fig. 1B). Significant differences in the overall proportions were observed between the healthy control group and the cirrhotic group. We confirmed that the abundance of Bacteroidetes decreased (P < 0.0001); conversely, the abundance of Proteobacteria (P < 0.0001) and Actinobacteria (P = 0.033) increased in the fecal samples from the cirrhosis group with or without HE (Fig. 1A). In addition, at the family level, Rumunococcaceae (P < 0.0001), Lachnospiraceae (P = 0.0007), and Prevotellaceae (P < 0.0001) were depleted, but Enterococcaceae (P = 0.052) and Lactobacillaceae (P = 0.0065) levels were elevated (Supplementary Fig. 1A). Alpha diversity was determined based on the Chao1, ACE and Shannon metrics (Fig. 1B and Supplementary Fig. 1C). Compared to the healthy control group, all the cirrhotic groups exhibited significant decreases (P < 0.01) in species richness (Chao1 and ACE) and diversity indices (Shannon). In terms of differences between the cirrhotic groups with and without HE, a decrease in the diversity indices of the in cirrhotic patients with HE was observed (Chao1, P = 0.0014; ACE, P = 0.0009; Shannon, P = 0.025). We confirmed the abundance of specific taxa according to differences in liver diseases (Fig. 1C). Ruminococcaceae (P < 0.0001) and Lachnospiraceae (P < 0.0001) were significantly decreased in fecal samples from the cirrhotic patient group. Moreover, we also analyzed which species varied by patient group, and particular strain in the gut microbiota was A. muciniphila (P = 0.0039).
Cirrhotic patients with hepatic encephalopathy and cognitive impairment patients exhibited a decreased fecal bacterial abundance of Akkemansia muciniphila, and which was also identified in animal models. a Taxa summary of bacterial phyla level obtained by 16S rDNA sequencing of fecal samples. b Microbial alpha diversity Chao1 in the context of disease progression classifications. **P < 0.01; Wilcoxon rank-sum test. c Relative abundances of species with significantly different representations in human groups. Data represent the means ± SEM; **P < 0.01; one-way ANOVA. d Relative abundance of bacterial phyla level obtained by 16S rDNA sequencing of fecal samples. e Microbial alpha diversity in patients with cognitive impairment. f Relative abundances of A. muciniphila in healthy control and cognitive impairment group. Data represent the means ± SEM; *P < 0.05, unpaired t-test. g Pearson’s correlation coefficients, p values, and linear relationships of A. muciniphila relative abundance (%) and MMSE-KC score. h Schematic of intervention with DDC diet (red) during the 3 weeks of 4 weeks DDC-induced liver injury animal model. i Relative abundance of phylum, family and A. muciniphila in control mice and DDC diet mice. AKK, Akkermansia muciniphila; AKKp, pasteurized Akkermansia muciniphila. Data represent the means ± SEM; n ≥ 3; *P < 0.05; unpaired t-test
We performed a 16S rRNA gene sequencing analysis of fecal samples from patients with cognitive impairment to understand the cognitive impairment symptoms in cirrhotic patients. No significant differences in alpha diversity or taxon proportions at the phylum and family levels were detected between the healthy control and cognitive impairment groups (Fig. 1D and E, Supplementary Fig. 1D, and 1E). Interestingly, we observed that the abundance of A. muciniphila was reduced (P = 0.032) in the cognitive impairment patient group but not in the healthy control group, which was consistent with the 16S rRNA sequencing results from the fecal samples of the cirrhotic patients (Fig. 1F). Hence, a positive correlation (R = 0.015; P = 0.09) was observed between the abundance of A. muciniphila and the MMSE-KC score, which is a criterion for cognitive impairment (Fig. 1G). In linear discriminant effect size analysis (LEfSe) performed to confirm differences in bacterial community composition between groups, differences in A. muciniphila according to disease were commonly identified in both models (Supplementary Fig. 2).
To demonstrate the link between human liver disease phenomena and gut dysbiosis, we investigated animal models of liver disease in mice orally administered 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (Fig. 1H). A gut microbiome imbalance was observed at the phylum and family levels, and for specific species, the abundance of A. muciniphila decreased (P = 0.07) with the progression of liver damage through the consumption of the DDC diet (Fig. 1I). These results indicate that the abundance of A. muciniphila in the gut is likely related to cognitive impairment and the progression of liver injury and may be an important factor. Interestingly, in an animal model of oral DDC administration that mimics human liver disease, similar cirrhotic symptoms and cognitive impairment were observed. Furthermore, a histological evaluation of liver tissue using H&E staining revealed that hepatitis was significantly increased (P = 0.018) in the DDC diet group. (Supplementary Fig. 3A and 3B). Notably, we detected neuronal cell death in the hippocampus associated with cognitive impairment in animal models of DDC-induced hepatocellular injury using IHC staining (Supplementary Fig. 3C and 3D). We detected damage to the enteric nerve layer with the PGP 9.5 antibody, which stains enteric neuronal cells (Supplementary Fig. 3E and 3F). Additionally, cognitive deficits due to hippocampal neuronal death were observed in the DDC diet group (Supplementary Fig. 3G). Through these results, we successfully established an animal model of liver disease and identified A. muciniphila depletion, consistent with the neuropsychiatric abnormalities observed in human patients.
Protective effects of A. muciniphila on gut inflammation and hepatocellular injury in a DDC diet animal model
We administered live or pasteurized A. muciniphila to C57BL/6 mice fed the DDC diets for 8 weeks to investigate the protective effect of A. muciniphila on liver damage, (Supplementary Fig. 4A). Afterward, we checked the plasma ALT and total bilirubin levels in the mice, which were consequently decreased in the DDC + A. muciniphila– and DDC + pasteurized A. muciniphila-treated groups compared to the DDC group (Fig. 2A). The effect of A. muciniphila on alleviating liver injury was confirmed by H&E and Masson’s trichrome staining (Fig. 2B and C). Additionally, an increase in apoptosis in the DDC model was observed using TUNEL staining, confirming the effect of treatment with A. muciniphila or pasteurized A. muciniphila on decreasing apoptosis (Fig. 2D and E). The mRNA expression of liver genes (Col1a1, Tgfβ, Timp1, and α-sma) was also downregulated in both the DDC + A. muciniphila– and DDC + pasteurized A. muciniphila-treated groups compared to the DDC-only-treated group (Fig. 2F). The protective effect of A. muciniphila on enteric nerve layer damage was confirmed by H&E staining (Fig. 2G).
Oral administration of A. muciniphila alleviates liver injury and protects intestinal barrier in a mouse model. a Plasma ALT and total bilirubin levels. Data represent the means ± SEM; n ≥ 4; **P < 0.01, *P < 0.05; one-way ANOVA. b, c Histological assessment of liver injury with representative images of H&E and Masson’s trichrome stained liver sections. Data represent the means ± SEM; n ≥ 3; **P < 0.01, *P < 0.05; one-way ANOVA. Scale bar, 200 μm. d, e TUNEL staining in the liver from mouse. Data represent the means ± SEM; n ≥ 3; **P < 0.01 one-way ANOVA. Scale bar, 50 μm. f Hepatic mRNA expression of liver genes (Col1a, Tgfβ, Timp1, and α-sma). Data represent the means ± SEM; n ≥ 4; **P < 0.01, * P < 0.05; one-way ANOVA. g Histological assessment of colon crypt with representative images of H&E stained colon sections. Data represent the means ± SEM; n ≥ 3; **P < 0.01, *P < 0.05; one-way ANOVA. Scale bar, 200 μm. h GI tract dysfunction characterization measured by the colon length. Data represent the means ± SEM; n = 3; *P < 0.05; one-way ANOVA. i Immunohistochemical staining of phosphorylated NFκB in the mouse colon. Data represent the means ± SEM; n ≥ 3; ****P < 0.0001 one-way ANOVA. Scale bar, 200 μm. j Colon mRNA expression of tight junction-related genes (Zo-1, Occludin). Data represent the means ± SEM; n ≥ 4; **P < 0.01, * P < 0.05; one-way ANOVA. k Plasma FD4 (fluorescein isothiocyanate dextran 4) level of the in vivo gut permeability assay. Data represent the means ± SEM; n ≥ 4; **P < 0.01; one-way ANOVA
We also measured the shortening of the colon length, which is a measure of gut inflammation, and shortening symptoms were alleviated in both the DDC + A. muciniphila– and DDC + pasteurized A. muciniphila-treated groups (Fig. 2H). Additionally, the level of phosphorylated nuclear factor kappa B (pNF-kB) which is a hallmark of chronic inflammatory diseases, in the mouse colon was measured using IHC staining, and it was significantly reduced in both the DDC + A. muciniphila– and DDC + pasteurized A. muciniphila-treated groups (Fig. 2I). The mRNA expression of the proinflammatory cytokine Tnfα was downregulated in plasma. Additionally, a significant decrease (P < 0.05) in the TNFα concentration was detected in liver tissue lysate samples. (Supplementary Fig. 4B).
We measured the mRNA expression levels of the tight junction proteins Zo-1 and Occludin in mouse colon tissue to evaluate whether A. muciniphila prevents gut leakage. The results showed that the administration of DDC led to a decrease in the mRNA expression of the tight junction proteins Zo-1 and Occludin in colonic tissue, which was restored by A. muciniphila administration (Fig. 2J). Fluorescein isothiocyanate-dextran 4 kDa (FD4) was applied to the intestinal mucosa and tracked systemically in the plasma to quantify gut leakiness, and A. muciniphila reduced the systemic translocation of FD4 (Fig. 2K). We also measured plasma lipopolysaccharide (LPS)/lipopolysaccharide-binding protein (LBP) concentrations and found that the administration of A. muciniphila reduced (P ≤ 0.05) the plasma endotoxin concentrations (Supplementary Fig. 4C).
Furthermore, animal models of liver disease were created through bile duct ligation (BDL) surgery to determine the efficacy of A. muciniphila in various animal models of liver injury (Supplementary Fig. 5A). We administered A. muciniphila to the BDL surgery model, which causes hepatocellular injury and liver cell apoptosis, and it subsequently alleviated liver injury (Supplementary Fig. 5B). Similarly, the mRNA expression levels of liver genes (Col1a1, Timp1, and α-sma) were lower in mice administered A. muciniphila than in those in the BDL surgery-only group (Supplementary Fig. 5C). On the other hand, the mRNA expression level did not changed.
Reduction in gut inflammation and enteric neuronal cell death by A. muciniphila in a DDC diet animal model
We analyzed serotonin level in gut tissues through IHC staining using serotonin-specific antibodies to identify enteric neuronal cell death and gut inflammation in an animal model of liver disease generated by oral administration of DDC. We observed a significant decrease in 5-HT (5-hydroxytryptamine) expression in the enteric neuronal system (ENS) in the DDC-treated group compared to the control chow group. However, in the group treated with A. muciniphila and pasteurized A. muciniphila, we observed increased 5-HT expression. We also validated the improvement in gut leakage through structural analysis of colon tissue sections based on isolation of the enteric nerve layer (Fig. 3A and B). To assess whether DDC regulates Iba-1, PGP 9.5 (enteric neural marker) and 5-HT expression in the colon, we performed 5-HT/Iba-1 or Iba-1/PGP9.5 co-immunofluorescence staining. In the DDC treatment group, the amount of 5-HT decreased and the expression of Iba-1 which indicates activated microglia increased compared that in the control group, but the inflammatory response was significantly improved in the group treated with A. muciniphila or pasteurized A. muciniphila in combination with DDC (Fig. 3C). The graph quantifying the results below the representative image confirms this finding (Fig. 3E). Furthermore, the increase in Iba-1 expression was accompanied by a decrease in PGP9.5 expression in the DDC-treated group, and an increase in PGP9.5 expression was observed in the group treated with A. muciniphila or pasteurized A. muciniphila, confirming that A. muciniphila mitigated gut leakage and inflammation (Fig. 3D and F). Remarkably, immunohistochemical staining of the vagus nerve with a 5-HT-specific antibody revealed high 5-HT expression in the A. muciniphila and pasteurized A. muciniphila treatment groups. This result provides direct evidence that A. muciniphila specifically regulates expression in serotonergic interneurons (Fig. 3G and H).
Serotonin deficiency and activation of neuroinflammatory mechanisms in the gut and brain axis of a liver disease animal model and neuronal cell deaths alleviated by A. muciniphila administration. a, b Immunohistochemistry staining of 5-HT in the colon of mice. Quantitative analysis of 5-HT-positive cells. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA. Scale bar, 100 μm. c Immunofluorescent staining of anti-5-HT/anti-Iba-1 in the colon of mice and the fluorescent signals were quantified (d) Immunofluorescent staining of anti-Iba-1/anti-PGP9.5 in the colon of mice and the fluorescent signals were quantified (e) Scale bar, 10 μm. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA. f Scale bar, 10 μm. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA. g, h Therapeutic effect of AKK or pasteurized AKK (AKKp) treated groups in the vagus nerve of mice were analyzed by immunohistochemistry staining. Scale bar, 20 μm. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA
Hippocampal neuronal cell death with cognitive impairment in an animal models of liver disease
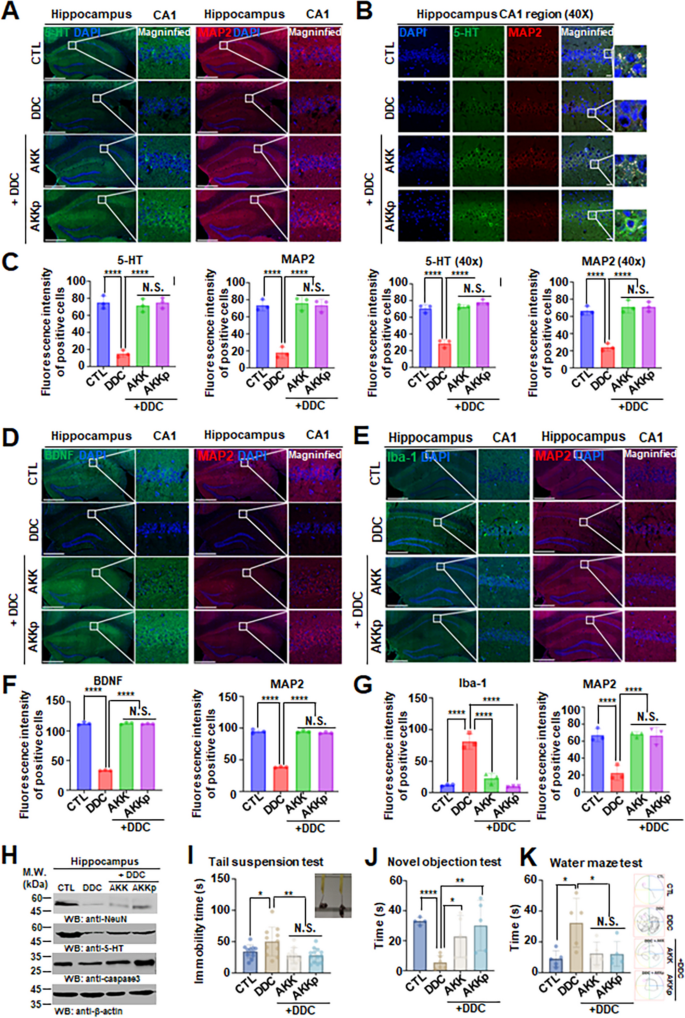
We measured serotonin levels and the expression of the the neuronal marker microtubule associated protein-2 (MAP2) in the hippocampal brain region to determine the importance of the gut-liver-brain axis in animal models of liver disease. Surprisingly, the expression of serotonin and MAP2 was significantly reduced in the group receiving DDC orally compared to the control group. Furthermore, the DDC-induced decrease in serotonin and MAP2 expression were reversed in the group treated with A. muciniphila and pasteurized A. muciniphila (Fig. 4A, B, and C). Moreover, to determine whether A. muciniphila also affects dopamine expression, we examined dopamine expression in the substantia nigra of the brain and in the gut and vagus ganglia and found that dopamine expression was reduced by DDC but was not restored by A. muciniphila (Supplementary Fig. 6A, S6B, and S6C). The vagus nerve is a single neuron that connects the gut-brain axis and is referred to as the highway for neurotransmitters produced in the brain and gut [28, 29].
Both BDNF and 5-HT are reduced in DDC only treated group hippocampus, while increased in A. muciniphila treated group. a, b, c Co-immunofluorescence staining analysis of anti-5-HT/anti-MAP2 in the hippocampus CA1 region of mice and the fluorescent signals (a, b) were quantified (c). Scale bar, 100 μm. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA. d, e, f, g The immunofluorescence co-staining of anti-BDNF/anti-MAP2 (d, f) and anti-Iba-1/anti-MAP2 (e, g) in the hippocampus of the above animals. Scale bar, 100 μm. Data represent the means ± SEM; representative data of 3 samples; ****P < 0.0001; N.S. = not significant, one-way ANOVA. h Western blot analysis of prefrontal cortex and hippocampus lysates from the animals treated with DDC only or both treated DDC + AKK group or DDC + AKKp group. i Immobility time graph of tail suspension test. Data represent the means ± SEM; n ≥ 11; *P < 0.05, **P < 0.01; N.S. = not significant, unpaired t-test. j Cognitive impairment tests of novel objection test and water maze test (k) were performed. Data represent the means ± SEM; n ≥ 4; *P < 0.05, **P < 0.01; N.S. = not significant, unpaired t-test
In addition, the levels of BDNF, a molecule that plays an important role in cognitive function in the brain, and Iba-1, a marker of activated microglia, were measured in each group. Similar to previous results, BDNF expression was reduced in the DDC-treated group (Fig. 4D), and Iba-1 expression was significantly increased (Fig. 4E). In contrast, quantitative and qualitative analyses confirmed that BDNF expression was increased and Iba-1 expression was decreased in DDC + A. muciniphila- or DDC + pasteurized A. muciniphila-treated groups (Fig. 4F and G). Similar observations were confirmed by immunoblotting. Increased neuronal death and 5-HT depletion was observed in the DDC-only group but the loss of hippocampal neurons and 5-HT was prevented in the DDC + A. muciniphila- or DDC + pasteurized A. muciniphila-treated groups (Fig. 4H). We also conducted animal behavioral experiments related to cognitive function and depression to determine the effects of neuronal death in the hippocampus and decreased expression of BDNF and serotonin and found that the DDC-treated group exhibited depression and cognitive dysfunction, which was improved in the A. muciniphila and pasteurized A. muciniphila-treated groups (Fig. 4I, J, and K). Additionally, the therapeutic effect of A. muciniphila was also confirmed in the BDL surgery model (Supplementary Fig. 6D and 6E). These scientific analyses suggest that the inflammatory mechanism and apoptosis of gut and brain neurons in animal models of liver disease may be regulated by A. muciniphila, which regulates BDNF and serotonin expression.
Sarpogrelate inactivates 5HT2A/2B receptors in liver tissue but does not affect the 5HT/5HT receptors in the gut or brain via the gut-organ axis
Sarpogrelate is a 5-HT2A and 2B receptor antagonist that has been shown to inhibit the action of serotonin in the body. In the liver, serotonin is synthesized and released by platelets and contributes to the development of liver injury and portal hypertension in patients with cirrhosis [30, 31]. To investigate the inflammatory mechanisms and cognitive dysfunction of brain serotonergic neurons ameliorated by increased serotonin secretion by A. muciniphila in combination with sarpogrelate, we treated the animal model of DDC-induced liver injury with sarpogrelate for 5 weeks (Supplementary Fig. 7A). Consequently, the effect of sarpogrelate on alleviating liver injury was confirmed by H&E staining (Supplementary Fig. 7B), and the mRNA expression of liver genes (Col1a, Tgfβ, Timp1, and α-sma) was also downregulated in the DDC + sarpogrelate + A. muciniphila– or DDC + sarpogrelate + pasteurized A. muciniphila-treated groups compared to the group treated without sarpogrelate (Supplementary Fig. 7C). However, we found that sarpogrelate had no effect on the inflammatory mechanisms and cognitive dysfunction, or serotonergic neurons in the brain (Supplementary Fig. 8). These results suggest the existence of a vagus ganglion-mediated gut-brain axis connection and a survival mechanism for serotonergic neurons in the hippocampal region of the brain that is mediated solely by nonblood neurotransmitters.
The administration of A. muciniphila alleviates liver injury by suppressing 5-HT2A/2B receptor expression
To investigate whether A. muciniphila relieves liver injury through serotonin receptors, we measured 5-HTR2A/2B expression levels in mouse liver tissue (Fig. 5A, C, E, and G). The expression of 5-HTR2A/2B was significantly increased in the group orally administered DDC compared to the control group. In addition, the increase in 5-HTR2A/2B expression induced by DDC was reduced in the groups treated with A. muciniphila and pasteurized A. muciniphila. The expression of the receptors was examined in the liver tissue of a hepatocellular injury mouse model treated with sarpogrelate to determine whether the 5-HTR2A/2B antagonist has an effect on A. muciniphila-induced expression reduction, (Fig. 5B, D, F, and H). Additionally, we confirmed whether 5-HTR2A/2B expression levels change in cell experiments. When the LX-2 human hepatic stellate cell line was treated with sarpogrelate or pasteurized A. muciniphila, the expression levels of 5-HTR2A/2B decreased, which was consistent with the results from the animal model (Supplementary Fig. 7D). Taken together, these results suggest that administration of A. muciniphila or pasteurized A. muciniphila can achieve the remission of liver injury by inhibiting 5-HTR2A/2B, which contributes to liver injury activity by binding to serotonin.
5-HT2A/2B receptor expression are increased in DDC only treated group liver tissue, while reduced in A. muciniphila treated group. a, b, c, d immunofluorescence staining analysis of anti-5-HTR2A in the liver tissue of mice and the fluorescent signals (a, b) were quantified (c, d). Scale bar, 100 μm. Data represent the means ± SEM; n ≥ 3; **P < 0.01, *P < 0.05; one-way ANOVA. e, f, g, h immunofluorescence staining analysis of anti-5-HTR2B in the liver tissue of the above mice. The fluorescent signals (e, f) were quantified (g, h). Scale bar, 100 μm. Data represent the means ± SEM; n ≥ 3; ***P < 0.005, **P < 0.01; one-way ANOVA
Validation of the correlation between BDNF/serotonin levels and disease progression in human subjects with liver fibrosis and cirrhosis
Since A. muciniphila had a positive effect on the gut-liver-brain axis through the regulation of serotonin and the recovery of BDNF levels in the brain, gut and liver in the animal model of liver injury, we checked plasma serotonin levels in the animal model, which were markedly reduced by DDC and recovered by A. muciniphila (Supplementary Fig. 9A). Additionally, the expression of 5-HT2A/2B mRNA in mouse liver tissue was confirmed (Supplementary Fig. 9B). No significant difference was observed in 5-HT2A receptor expression, but the 5-HT2B receptor expression level was significantly decreased by pasteurized A. muciniphila.
We verified the changes in serotonin and BDNF according to the exacerbation of liver cirrhosis by analyzing portal blood and liver biopsies from human subjects (Fig. 6). Biopsy samples from patients with liver disease confirmed the mRNA expression of the 5-HT2A/2B receptor and BDNF (Fig. 6A). The expression of the 5-HT2A receptor was increased in patients with stage 2 hepatitis compared with patients with fatty liver disease (stage 1). However, a reduction was observed in stage 3 patients or patients with advanced fibrosis (cirrhosis). In contrast, the 5-HT2B receptor was activated and its levels were significantly increased in stage 3 patients and patients with severe cirrhosis. Similar observations were confirmed by immunoblotting. 5-HT2A receptor expression increased from stage 1 to stage 2 and decreased in stage 3. Additionally, 5-HT2B receptor expression increased as the stage progressed (Fig. 6A). We found that BDNF steadily decreases with the progression of liver disease, a pattern that was consistent with findings from patient with liver disease and various animal models of liver disease (Fig. 6B). The hepatic portal blood we sampled was associated with portal hypertension, a common symptom in patients with hepatopathy, which allowed us to specifically identify the increase in serotonin associated with increased blood cells [32].
BDNF/serotonin is associated with the progression of liver cirrhosis. a mRNA expression of 5-HT2A receptor and 5-HT2B receptor, and BDNF in human liver biopsy. Western blot analysis of 5-HT2A receptor and 5-HTR2B receptor in human liver biopsy. Data represent the means ± SEM; n = 10; **P < 0.01, *P < 0.05. one-way ANOVA. b Concentrations of serotonin and BDNF measured in portal blood of patients with liver cirrhosis. Data represent the means ± SEM; n ≥ 12; **P < 0.01, *P < 0.05. one-way ANOVA. c Pearson’s correlation coefficients, P values, and linear relationships of serotonin and BDNF concentration, A. muciniphila relative abundance, and HVPG. d Pearson’s correlation coefficients, p values, and linear relationships of serotonin and BDNF concentration, A. muciniphila relative abundance, and Child–Pugh score
We confirmed the correlation between HVPG, serotonin, BDNF, and A. muciniphila abundance and validated the association with liver cirrhosis (Fig. 6C). A positive correlation was observed between serotonin concentrations and HVPG, whereas a negative correlation was observed with BDNF concentrations. In addition, a negative correlation was observed between the HVPG and the relative abundance of A. muciniphila, which was confirmed to have a positive effect on the liver and cognitive function in animal models. These results were the same even when the patient group was divided based on Child–Pugh score (Fig. 6D). Hence, the current results suggest that BDNF is another important molecule in addition to serotonin in human liver disease progression and cirrhosis.








Add Comment