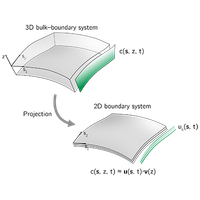
Intracellular protein patterns regulate many vital cellular functions, such as the processing of spatiotemporal information or the control of shape deformations. To do so, pattern-forming systems can be sensitive to the cell geometry by means of coupling the protein dynamics on the cell membrane to dynamics in the cytosol. Recent studies demonstrated that modeling the cytosolic dynamics in terms of an averaged protein pool disregards possibly crucial aspects of the pattern formation, most importantly concentration gradients normal to the membrane. At the same time, the coupling of two domains (surface and volume) with different dimensions renders many standard tools for the numerical analysis of self-organizing systems inefficient. Here, we present a generic framework for projecting the cytosolic dynamics onto the lower-dimensional surface that respects the influence of cytosolic concentration gradients in static and evolving geometries. This method uses a priori physical information about the system to approximate the cytosolic dynamics by a small number of dominant characteristic concentration profiles (basis), akin to basis transformations of finite element methods. As a proof of concept, we apply our framework to a toy model for volume-dependent interrupted coarsening, evaluate the accuracy of the results for various basis choices, and discuss the optimal basis choice for biologically relevant systems. Our analysis presents an efficient yet accurate method for analyzing pattern formation with surface-volume coupling in evolving geometries.
- Received 15 May 2024
- Accepted 26 July 2024
DOI:https://doi.org/10.1103/PhysRevE.110.034412
©2024 American Physical Society
Physics of Living SystemsNonlinear Dynamics



Add Comment