Citation: Alexander NG, Cutts WD, Hooven TA, Kim BJ (2024) Transcription modulation of pathogenic streptococcal and enterococcal species using CRISPRi technology. PLoS Pathog 20(9):
e1012520.
https://doi.org/10.1371/journal.ppat.1012520
Editor: Kimberly A. Kline, University of Geneva: Universite de Geneve, SWITZERLAND
Published: September 19, 2024
Copyright: © 2024 Alexander et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: B.J.K. is supported by the National Institute of Neurological Disorders and Stroke grant R15NS131921, and the National Institute of Allergy and Infectious Diseases grant R03AI185593 and received salary support from both grants. T.A.H. is supported by the National Institute of Allergy and Infectious Disease grants R21AI147511 and K08AI132555 and received salary support from both grants. The funders had no role in a study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
CRISPR, short for clustered regularly interspaced short palindromic repeats, is found in 40% of bacteria and 85% of archaea providing defense against phage and other foreign DNA [1–3]. The CRISPR locus contains both “spacers,” which encode guide RNAs (gRNAs) used to direct the system to target nucleic acids, and Cas effector proteins. There are several Cas effector proteins with some functioning as endonucleases, while others serve for recognition or processing of gRNA. Certain Cas effectors, such as Cas9, can be directed to cut a given site via recognition of a gRNA, creating a double-stranded break in the target DNA. These effectors can scan long sequences of DNA for short sequences called protospacer adjacent motifs (PAMs), before unraveling the DNA to cut. These PAMs vary between different CRISPR-Cas types [4,5].
CRISPR interference (CRISPRi) utilizes a catalytically dead Cas effector (dCas), through targeted mutation of the catalytic domains, specifically HNH and RuvC-like nuclease domains in Cas9, to eliminate Cas endonuclease activity [4,5]. This dCas effector retains its ability to recognize a gRNA and bind DNA. If guided to a gene, dCas will bind and form a “roadblock” on the gene, reducing transcription and causing a knockdown in expression of the gene as shown in Fig 1.
Fig 1. CRISPRi can be approached in a species-dependent manner, either utilizing an organism’s endogenous Cas effector protein or via the introduction of Spy dCas9.
Either of these approaches entails targeted mutagenesis of the HNH and RuvC-like nuclease domains responsible for Cas9’s cutting activity. If this catalytically dead Cas9 is endogenous, a plasmid can be transformed containing a protospacer that is expressed as a sgRNA targeted to a gene of interest to produce a knockdown. If using Spy dCas9 non-endogenously, the gene will either be chromosomally inserted into your target organism or expressed on a plasmid with the corresponding targeting protospacer. Fig 1 created in BioRender. CRISPR, clustered regularly interspaced short palindromic repeats; CRISPRi, CRISPR interference; dCas, dead Cas; sgRNA, single-guide RNA; Spy dCas9, S. pyogenes dCas9.
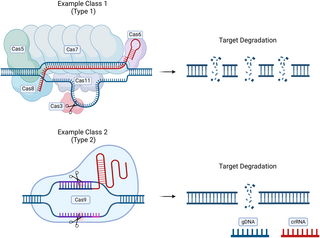
This can be most easily achieved in Class 2 Cas effectors (primarily Cas9 and Cas12a), as these rely primarily on a single effector for both target binding and DNA cleavage rather than the effector complexes of Class 1 systems, which feature a cascade of Cas proteins (shown in Fig 2) [6,7]. dCas transcriptional regulators allow for near-silencing of multiple genes making CRISPRi technology robust and high throughput. CRISPRi systems can be modified and applied to a variety of bacteria.
Fig 2. Class 1 and 2 CRISPR-Cas systems vary significantly, with class 2 systems being more user-friendly for CRISPRi.
Class 1 systems involve a cascade with a number of Cas proteins being utilized to achieve target recognition, binding, and targeted cleavage. Class 2 systems utilize a single effector such as Cas9 or Cas12 for target recognition, binding, and targeted cleavage. Fig 2 created in BioRender. CRISPR, clustered regularly interspaced short palindromic repeats; CRISPRi, CRISPR interference; crRNA, CRISPR RNA; gDNA, genomic DNA.
We will review recent examples of CRISPRi technology in Enterococcus spp., Streptococcus pyogenes, Streptococcus agalactiae, and Streptococcus pneumoniae. We will also discuss future applications of CRISPRi technology including direct genome editing, gene silencing, and CRISPRseq.
Enterococcus spp.
Enterococcus faecalis is a gram-positive opportunistic pathogen known to cause multidrug-resistant infections including sepsis, endontic infections, and hospital-acquired urinary tract infections [8–10]. E. faecalis possess a type-II CRISPR-Cas9 system. Interestingly, E. faecalis clinical isolates that are multidrug-resistant have mobile genetic elements that can be targeted by CRISPR-Cas systems and then maintained [11]. E. faecalis has shown that there is a phenotype of “CRISPR tolerance,” which corresponds to transient maintenance of targeted DNA [11].
Targeted mutagenesis through allelic exchange has been a frequently used method for genome editing. An alternative to this method was developed in E. faecalis, which utilizes a two-plasmid CRISPR-Cas9-mediated genome editing system that allows for a reduction of screening needed, as well as a shortened period for genome editing [12]. Another two-plasmid system was generated in Enterococcus faecium, utilizing inducible expression of RecT, an ssDNA-annealing protein associated with phage recombination, in combination with CRISPR-Cas9-based counterselection. This system permits either genomic insertion or deletion while minimizing screening, making it a rather versatile approach to mutagenesis [13]. Limitations of this system include reduced transformation efficiency due to the toxicity of Cas9, as well as the need for multiple large vectors [14].
An E. faecalis CRISPRi tool was created for use in high-throughput, scalable genetic control studies eliminating the limitation around reduced transformation results with the genome editing system. A dual-vector nisin-inducible system was tested and shown to silence both single genes and whole operons [15]. Additionally, the system was able to silence genes involved in biofilm formation and antibiotic resistance [15]. Future applications include investigating antibiotic resistance mechanisms and virulence factors associated with E. faecalis infection.
Streptococcuspyogenes
S. pyogenes is a gram-positive bacterium responsible for many infections including pharyngitis, necrotizing fasciitis, and toxic shock syndrome [16–18]. S. pyogenes can possess either CRISPR Type I-C or Type II-A.
S. pyogenes is notable for the Cas9 system known as Spy Cas9, which was utilized to develop CRISPRi [19]. The process entailed cloning the S. pyogenes Cas9 gene along with a minimal CRISPR locus into either an expression vector or the target organism’s genome. Spy Cas9 has been used extensively as a platform for producing CRISPRi functionality in bacteria that do not harbor an endogenous Cas9 [5,20].
While Spy Cas9 has been used to apply CRISPRi in other species, the same could not be said for S. pyogenes until recently. A study has shown successful implementation of a doxycycline-inducible dCas9-based CRISPRi system in S. pyogenes [21]. The doxycycline induction system permits tight expression regulation, and researchers were able to demonstrate that the system could reliably knock down target genes of interest. A library of unique spacers featuring near-complete coverage of the S. pyogenes genome was made accessible in SpyBrowse, the S. pyogenes genome browser. Future directions include the transformation and implementation of the spacers, and generation of a CRISPRi knockdown library.
Additionally, it has been demonstrated that in S. pyogenes strains with a type II-A CRISPR-Cas system, there are fewer prophages compared to type I-C CRISPR-Cas strains [22]. Interestingly, it was also shown that a unique streptococcal phage “anti-CRISPR,” a phage-encoded protein that interferes with CRISPR-Cas systems, can inhibit S. pyogenes Cas9 [23]. Future studies are needed to examine the role of anti-CRISPRs in other streptococcal species.
Streptococcusagalactiae
S. agalactiae is a gram-positive pathobiont that colonizes the genitourinary and gastrointestinal mucosa. S. agalactiae is capable of opportunistic vertical transmission to the neonate, which can result in meningitis, chorioamnionitis, or neonatal sepsis [24,25]. Additionally, S. agalactiae is also a rare cause of infection in nonpregnant adults [26,27]. S. agalactiae, like S. pyogenes, harbors either a Type-I-C or Type-II-A CRISPR system, which has been exploited to produce CRISPRi strains like those generated in S. pyogenes. Notably, the residues associated with Cas9 catalytic activity do vary from S. pyogenes, with the HNH nuclease domain shifted such that the point mutation associated with catalytic inactivation is H845A rather than H840A [19,28].
CRISPRi has been applied as a tool for the generation of rapid, flexible knockdown phenotypes [28,29]. CRISPRi has also been utilized to investigate the various roles that the CRISPR/Cas system plays apart from its canonical role as a nuclease, highlighting a potential role in regulating virulence, which has been suggested in previous non-CRISPRi studies [28,30]. There has been a recent surge in the development of CRISPRi technologies for S. agalactiae. A group has developed multiple dCas9 strains for use in exploring S. agalactiae transcription and fitness [28,29]. These developments can be expanded on for future research, with the potential for added complexity using sgRNA multiplexing.
Streptococcuspneumoniae
S. pneumoniae is a gram-positive pathobiont colonizing the upper respiratory mucosa that can cause pneumonia, meningitis, or otitis media [31]. S. pneumoniae does not possess an endogenous CRISPR/Cas system, but the natural competence and relative ease of genetic manipulation simplifies most CRISPRi-based applications. One common approach is the chromosomal insertion of Spy dCas9 and a single-guide RNA (sgRNA), allowing for expression. Additionally, this system can be driven by an inducible promoter, allowing for greater experimental complexity [32,33].
In S. pneumoniae, CRISPRi has been applied in combination with high-throughput Tn-seq (transposon sequencing) [34]. Tn-seq-based essential gene identification can be followed up with CRISPRi screening to identify previously unexplored genes. Tn-seq has been utilized in S. pneumoniae strain D39 for the identification of genes potentially related to antibiotic resistance [33]. Another application of CRISPRi is CRISPRi-seq and its notable subtype sCRilecs-seq. CRISPRi-seq is an approach that allows for minimal off-target effects through careful targeting of all operons rather than individual genes [32]. sCRilecs-seq (subsets of CRISPR interference libraries extracted by fluorescence-activated cell sorting coupled to next-generation sequencing) is a process that couples CRISPRi with fluorescence-activated cell sorting (FACS) to identify loci associated with a given phenotype [35]. Bacteria with desired fluorescence intensities can be collected and subject to sequencing to determine identity. This technique has been used to identify pathways that could function as combination treatment targets to deal with the growing threat of ß-lactam resistance in S. pneumoniae [36]. The ease of mutagenesis in S. pneumoniae facilitates the utilization of complex screening techniques, allowing CRISPRi technology to continue to expand and provide valuable results.
Future directions in CRISPRi technology
CRISPRi is a growing technology, with recent developments across different bacteria. The strengths of CRISPRi motivate continued development of screening technology as rapid, targeted expression manipulation is highly versatile. The push to develop CRISPRi technology in S. agalactiae is notable, yielding insights into transcription and fitness. Additionally, advanced applications such as SCRilecs-seq and CRISPRi-seq in S. pneumoniae or the nisin-inducible system developed for E. faecalis allow for increasingly complex and specialized investigations into antibiotic resistance mechanisms and virulence associated genes [15,32].
A future direction of interest would be utilization of Mobile CRISPRi, a technique developed for Pseudomonas aeruginosa. Mobile-CRISPRi allows for the transfer and integration of CRISPRi into bacteria through an antibiotic resistance cassette, sgRNA spacers, and a promoter that drives dCas9 expression [37]. CRISPRi technology is expanding and continues to demonstrate exciting results as it is used in a wider span of bacterial species. Additionally, CRISPRi may be of significant value to agricultural fields requiring scalable and functional investigation into target genes [38,39].
References
- 1.
Ledford H. CRISPR tools found in thousands of viruses could boost gene editing. Nature. 2022;612(7938):21. pmid:36418881 - 2.
Garneau JE, Dupuis M-È, Villion M, Romero DA, Barrangou R, Boyaval P, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468(7320):67–71. pmid:21048762 - 3.
Karginov FV, Hannon GJ. The CRISPR System: Small RNA-Guided Defense in Bacteria and Archaea. Mol Cell. 2010;37(1):7–19. pmid:20129051 - 4.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. pmid:22745249 - 5.
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–1183. pmid:23452860 - 6.
Yao R, Liu D, Jia X, Zheng Y, Liu W, Xiao Y. CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synth Syst Biotechnol. 2018;3(3):135–149. pmid:30345399 - 7.
Swarts DC, Jinek M. Cas9 versus Cas12a/Cpf1: Structure–function comparisons and implications for genome editing. WIREs RNA. 2018;9(5):e1481. pmid:29790280 - 8.
Kayaoglu G, Ørstavik D. Virulence Factors of Enterococcus faecalis: Relationship to Endodontic Disease. Crit Rev Oral Biol Med. 2004;15(5):308–320. pmid:15470268 - 9.
Madrazo M, Esparcia A, Alberola J, Ferrer A, Eiros JM, Nogueira JM, et al. Predictive factors for Enterococcus faecalis in complicated community-acquired urinary tract infections in older patients. Geriatr Gerontol Int. 2020;20(3):183–186. - 10.
Brown AO, Singh KV, Cruz MR, Kaval KG, Francisco LE, Murray BE, et al. Cardiac Microlesions Form During Severe Bacteremic Enterococcus faecalis Infection. J Infect Dis. 2021;223(3):508–516. - 11.
Hullahalli K, Rodrigues M, Nguyen UT, Palmer K. An Attenuated CRISPR-Cas System in Enterococcus faecalis Permits DNA Acquisition. MBio. 2018;9(3):e00414–e00418. - 12.
de Maat V, Stege PB, Dedden M, Hamer M, Jan P, Willems RJL, et al. CRISPR-Cas9-mediated genome editing in vancomycin-resistant Enterococcus faecium. FEMS Microbiol Lett. 2019;366(22). - 13.
Chen V, Griffin ME, Maguin P, Varble A, Hang HC. RecT Recombinase Expression Enables Efficient Gene Editing in Enterococcus spp. Appl Environ Microbiol. 2021;87(18):e00844–e00821. - 14.
Chua MJ, Collins J. Rapid, Efficient, and Cost-Effective Gene Editing of Enterococcus faecium with CRISPR-Cas12a. Microbiol Spectr. 2022;10(1):e0242721. - 15.
Afonina I, Ong J, Chua J, Lu T, Kline KA. Multiplex CRISPRi System Enables the Study of Stage-Specific Biofilm Genetic Requirements in Enterococcus faecalis. MBio. 2020;11(5): pmid:33082254 - 16.
Gaworzewska E, Colman G. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988;100(2):257–269. - 17.
Brouwer S, Rivera-Hernandez T, Curren BF, Harbison-Price N, De Oliveira DMP, Jespersen MG, et al. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat Rev Microbiol. 2023;21(7):431–447. - 18.
Brouwer S, Barnett TC, Rivera-Hernandez T, Rohde M, Walker MJ. Streptococcus pyogenes adhesion and colonization. FEBS Lett. 2016;590(21):3739–3757. - 19.
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8(11):2180–2196. pmid:24136345 - 20.
Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol. 2017;2(4):16274. pmid:28165460 - 21.
Bjånes E, Stream A, Janssen AB, Gibson PS, Bravo AM, Dahesh S, et al. An efficient in vivo-inducible CRISPR interference system for group A Streptococcus genetic analysis and pathogenesis studies. MBio. 2024;15(8):e0084024. - 22.
Yamada S, Shibasaki M, Murase K, Watanabe T, Aikawa C, Nozawa T, et al. Phylogenetic relationship of prophages is affected by CRISPR selection in Group A Streptococcus. BMC Microbiol. 2019;19(1):24. - 23.
Hynes AP, Rousseau GM, Lemay M-L, Horvath P, Romero DA, Fremaux C, et al. An anti-CRISPR from a virulent streptococcal phage inhibits Streptococcus pyogenes Cas9. Nat Microbiol. 2017;2(10):1374–1380. - 24.
Horiba K, Suzuki M, Tetsuka N, Kawano Y, Yamaguchi M, Okumura T, et al. Pediatric sepsis cases diagnosed with group B streptococcal meningitis using next-generation sequencing: a report of two cases. BMC Infect Dis. 2021;21(1):531. pmid:34090359 - 25.
Tavares T, Pinho L, Bonifácio AE. Group B Streptococcal Neonatal Meningitis. Clin Microbiol Rev. 2022;35(2):e0007921. pmid:35170986 - 26.
Sunkara B, Bheemreddy S, Lorber B, Lephart PR, Hayakawa K, Sobel JD, et al. Group B Streptococcus infections in non-pregnant adults: the role of immunosuppression. Int J Infect Dis. 2012;16(3):e182–e186. - 27.
Skoff TH, Farley MM, Petit S, Craig A, Schaffner W, Gershman K, et al. Increasing Burden of Invasive Group B Streptococcal Disease in Nonpregnant Adults, 1990–2007. Clin Infect Dis. 2009;49(1):85–92. pmid:19480572 - 28.
Gopalakrishna KP, Hillebrand GH, Bhavana VH, Elder JL, D’Mello A, Tettelin H, et al. Group B Streptococcus Cas9 variants provide insight into programmable gene repression and CRISPR-Cas transcriptional effects. Commun Biol. 2023;6(1):620. - 29.
Dammann AN, Chamby AB, Catomeris AJ, Davidson KM, Tettelin H, van Pijkeren J-P, et al. Genome-Wide fitness analysis of group B Streptococcus in human amniotic fluid reveals a transcription factor that controls multiple virulence traits. PLoS Pathog. 2021;17(3):e1009116. - 30.
Spencer BL, Deng L, Patras KA, Burcham ZM, Sanches GF, Nagao PE, et al. Cas9 Contributes to Group B Streptococcal Colonization and Disease. Front Microbiol. 2019;10:1930. pmid:31497003 - 31.
Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–367. - 32.
Liu X, Kimmey JM, Matarazzo L, De Bakker V, Van Maele L, Sirard J-C, et al. Exploration of Bacterial Bottlenecks and Streptococcus pneumoniae Pathogenesis by CRISPRi-Seq. Cell Host Microbe. 2021;29(1):107–20.e6. - 33.
Liu X, Gallay C, Kjos M, Domenech A, Slager J, Van Kessel SP, et al. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol Syst Biol. 2017;13(5):931. - 34.
Jana B, Liu X, Dénéréaz J, Park H, Leshchiner D, Liu B, et al. CRISPRi–TnSeq maps genome-wide interactions between essential and non-essential genes in bacteria. Nature Microbiology. 2024. pmid:39030344 - 35.
Gibson PS, Veening J-W. Gaps in the wall: understanding cell wall biology to tackle amoxicillin resistance in Streptococcus pneumoniae. Curr Opin Microbiol. 2023;72:102261. - 36.
Dewachter L, Dénéréaz J, Liu X, De Bakker V, Costa C, Baldry M, et al. Amoxicillin-resistant Streptococcus pneumoniae can be resensitized by targeting the mevalonate pathway as indicated by sCRilecs-seq. Elife. 2022;11:e75607. - 37.
Qu J, Prasad NK, Yu MA, Chen S, Lyden A, Herrera N, et al. Modulating Pathogenesis with Mobile-CRISPRi. J Bacteriol. 2019;201(22):e00304–19. pmid:31481541 - 38.
Zhu H, Li C, Gao C. Applications of CRISPR–Cas in agriculture and plant biotechnology. Nat Rev Mol Cell Biol. 2020;21(11):661–677. pmid:32973356 - 39.
Rao MJ, Wang L. CRISPR/Cas9 technology for improving agronomic traits and future prospective in agriculture. Planta. 2021;254(4):68. pmid:34498163





Add Comment