A facial microbiome genome compendium that better represented our and public facial metagenomic datasets through multi-center sampling and deep-sequencing
To enhance our understanding of the human facial microbiome and its association with aging and the physio-optical conditions of the skin, we conducted a study involving 479 healthy Chinese individuals of both genders and various ages (ranging from 18 to 64) from Beijing, Wuhan, and Guangzhou in China. These regions represent the Northern, Central, and Southern parts of China, respectively (Table S1). Inclusion of these regions is beneficial to the broad representation of constructed metagenome-assembled genomes (MAGs). We collected 498 skin samples from both cheeks of the volunteers, subjected them to deep metagenomic sequencing (Fig. 1A). After eliminating vector sequences and low-quality bases, we obtained an average of 102 million read pairs per sample. We estimated that 71.35% of these reads (interquartile range (IQR): 63.20 ~ 85.24%) were attributed to human DNAs, consistent with findings from previous studies [5]. Subsequent removal of host contaminations yielded an average of 29 million read pairs per sample. To mitigate potential impacts induced by physiological characteristics and personal lifestyle habits, we assess the physio-optical features of participants’ facial skin, including moisture, sebum, gloss, skin elasticity, and sensitivity (Tivi), using state-of-the-art equipment (“Methods” section), while relevant lifestyle information from the participants were collected (Table S1).
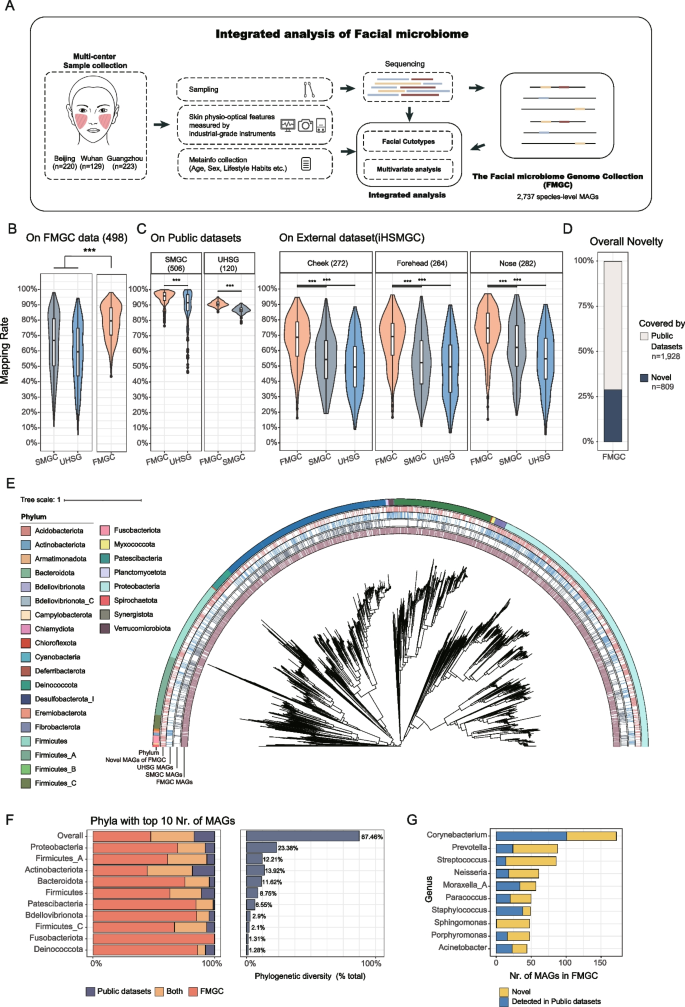
Construction and analysis of the Facial Microbiome Genome Compendium (FMGC). A Overall workflow of this study including the multi-center sample and meta info collection, measurement of skin physio-optical properties using industrial-grade instruments such as Corneometer, pH meter, Glossymeter, Sebumeter, and Cutometer from Courage and Khazaka, Visia-CR from Canfield and Tivi700 from WheelsBridge, generation of a representative Facial Microbiome Genome Compendium (FMGC) consisting 2737 species level genomes and integrative analyses of the (meta)genomic features with the host meta-data and skin features. B–D Mapping rates comparison: Comparison of mapping rates of facial metagenome sequencing reads to MAGs from FMGC, SMGC, and UHSG. The facial metagenome data included B 498 sample from this study; C 506 sample from SMGC study and randomly selected 120 sample from UHSG study; and 822 sample including 3 sample sites from the iHSMGC study [5]. D Novelty assessment: Evaluation of FMGC MAGs’ novelty based on their overlap with reference microbial genomes in the SMGC and those used by GTDB-tk and metaPhlAn4. E Phylogenetic analysis of the non-redundant 3359 MAGs combined from the FMGC and SMGC genomes. The orange color strip for FMGC MAGs, grey for SMGC MAGs, and blue for UHSG MAGs, and light pink for novel MAGs in FMGC. The outer ring is colored by phyla that were annotated using GTDB-tk [33]. F Phylogenetic diversity (branch length of selected MAGs) expansion of the FMGC over the SMGC genomes at the phylum level. The top ten 10 phyla with the highest number of MAGs were selected. G Expansion of the FMGC MAGs over the SMGC genomes at the genus level. Top 10 genera with the largest number of MAGs were selected. Statistical significance indicated by: ns p > 0.05, p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test
A comprehensive skin reference microbial genome catalogue is essential to characterize our samples. Recent studies have generated several such catalogs, including the SMGC [31] and the UHSG [32] catalog. SMGC was assembled from a total of 2479 skin metagenome samples including 506 facial samples and 1973 from other body sites, while UHSG used a total of 450 facial and 2069 other skin metagenomes. We thus evaluated their representativeness on our dataset by aligning our clean reads to these catalogs and calculated the mapping rates. Surprisingly, only 66.9% and 59.4% of our reads could be mapped to the two catalogs, respectively. Additionally, metaPhlAn4 [34], a widely used taxonomic profiling tool, reported ~ 45% unaccounted abundances by existing reference genomes (Fig. S1). Therefore, we have made the decision to assemble our own MAG as the basis to enhance the annotation and characterization of our data.
To construct our own set of MAGs, we employed a bioinformatics pipeline similar to SMGC [31] (“Methods” section). Briefly, we filtered out MAGs that were ≤ 200 kbp or deemed low-quality, high-contamination, or both based on CheckM [35] and de-replicated the remaining MAGs at an average nucleotide identity (ANI) of 95% (“Methods” section). We finally generated a comprehensive set of 2737 non-redundant species-level MAGs and referred it as the Facial Microbiome Genome Compendium (FMGC) (Table S2).
As expected, the FMGC genomes better represented our facial microbiome samples by recruiting a median of 80.0% sequencing reads, significantly higher than the public catalogues (p < 0.001, Wilcoxon rank sum test, Fig. 1B). Strikingly, FMGC also better represented available public facial microbiome datasets. For example, on the SMGC facial samples (n = 506), FMGC recruited 95.8% of the metagenomic sequencing reads, significantly higher than that by UHSG (91.3%, p < 0.001, Wilcoxon rank sum test; Fig. 1C). Similarly, FMGC recruited 90.37% of the sequencing reads of the UHSG facial samples (n = 120), higher than SMGC (~ 86.61%, p < 0.001, Wilcoxon rank sum test; Fig. 1C). For fair comparison, we conducted the reads recruitment using the three above mentioned catalogs on an independent public dataset used by the integrated Human Skin Microbial Gene Catalog (iHSMGC) [5]. FMGC recruited 68.5% of the sequencing reads of the iHSMGC samples (n = 822), higher than the SMGC (56.3%) and UHSG (50.98%, p < 0.001, Wilcoxon rank sum test; Fig. 1C).
As the iHSMGC dataset encompassed facial samples from multiple regions, while FMGC only collected from the cheek, we also examined whether the representativeness of the FMGC varied across different facial locations. The results revealed no significant differences in the representativeness of FMGC among the cheeks, forehead, and nose regions. We further conducted principal coordinate analysis (PCoA) on the iHSMGC samples, employing FMGC for abundance calculations to evaluate the overall microbial structure across these three facial regions. The results demonstrated a lack of significant differences among these areas (Fig. S2, p = 0.7), underscoring the consistent representativeness of FMGC across distinct facial regions.
To quantify the novelty in FMGC genomes to facial microbiome, we compared the genomic sequences with those in public reference skin microbial catalogs. At an ANI threshold of 95%, 752 and 807 of the FMGC MAGs could be found in SMGC and UHSG, respectively, totaling 1029 MAGs (Fig. S3A). In addition, 1644 and 1014 could also be found in the reference microbial genomes used by GTDB-tk [33] and MethPhlAn4 [34] at such an ANI threshold. Specifically, using GTDB-tk [33], we assigned all FMGC MAGs to either bacteria (n = 2734) or archaea (n = 3) at the kingdom level, 94–100% of them to known genus (94.26%) and higher taxonomic levels, and ~ 60% to known species (Table S1; Fig. S3B). Together, a total of 809 MAGs were considered as novel, representing 30% of the FMGC genomes (Fig. 1D and S3B). Importantly, these novel MAGs exhibited even higher qualities in genome size, N50 length and completeness while maintaining the same contamination level (~ 2.58%) as compared with those overlapping with the aforementioned datasets (Fig. S4; Wilcoxon rank sum test). These findings suggest that the novel MAGs likely represent genuine novel genomes rather than low-quality fragments resulting from mis-assembly.
We also quantified the contribution of FMGC to the increased genome diversity at different taxonomic levels. To achieve this, we merged all species-level MAGs from FMGC, SMGC, and UHSG, and obtained a total of 4167 MAGs. Remarkably, FMGC MAGs exhibited an expansive representation across diverse branches in the phylogenetic tree (Fig. 1E), in contrast to certain branches in public datasets that lacked representation (Fig. S5). In total, the FMGC contributed to a 47% increase in phylogenetic diversity throughout the entire tree (Fig. S5). Further examination at the phylum level revealed that most phyla displayed similar levels of increase (Fig. 1F). Similar trends were observed at the genus level (Fig. 1G). Notably, the Sphingomonas genus of the Proteobacteria phylum stands out, with 47 out of 48 MAGs exclusively detected in FMGC and only one MAG found in existing datasets (Fig. 1G).
Together, FMGC demonstrated a higher representativeness compared to existing public catalogues, contributing to a substantial increase in phylogenetic diversity across various taxonomic levels.
FMGC reveals two consistent cutotypes in facial samples across regions
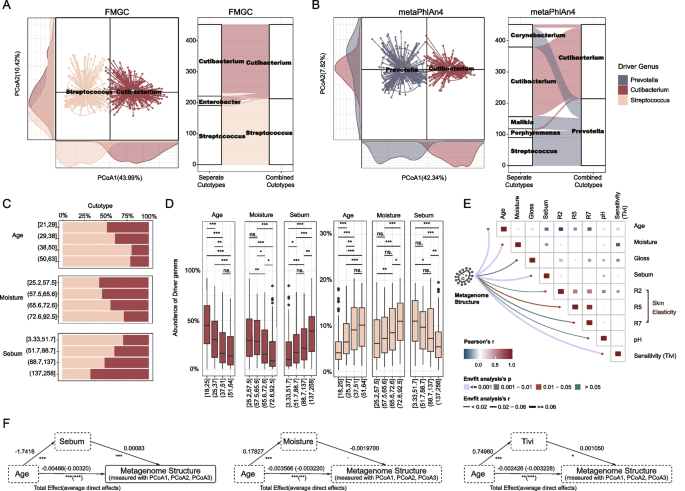
Cutotypes offer a comprehensive view of the inter-individual variation in facial microbial composition [5, 36]. A cutotype is a classification or grouping of facial microbiome samples (or based on the microbial composition into different skin microbiome profiles or community types. Community types in the gut bacteriome and mycobiome are associated with disease risks [37] and aging [38]. Similarly, we would expect that cutotypes can also help researchers to better understand the role of the skin microbiome in maintaining health and preventing skin-related issues. To identify and characterize cutotypes in our samples, we calculated the relative abundances at the genus-level by aligning the sequenced reads to the FMGC MAGs using BWA (“Methods” section). Genus-level abundances were chosen because community types are commonly determined at this level [39, 40], and the GTDB-tk tool we used for taxonomic classification could assign most of our MAGs (94.26%) to known genera (Fig. S6), ensuring the reliability of our results. Applying the Partitioning Around Medoids (PAM) clustering algorithm (“Methods” section), we obtained two cutotypes: the Cutibacterium-cutotype (C-cutotype) and Streptococcus-cutotype (S-cutotype; Fig. 2A). Importantly, we consistently observed these cutotypes when analyzing samples from individual regions or when combining them (Fig. 2A). And only a small fraction of samples (~ 6.2%) exhibited an additional cutotype, namely Enterobacter-cutotype.
Two consistent cutotypes built with FMGC and the correlation between microbial community structure and aging and skin physio-optical properties. Cutotypes constructed using genus-level abundances derived from the A FMGC MAGs and B metaPhlan4. The PCoA plots display the cutotypes built with all samples together. The Sankey diagram illustrates the analysis of samples separated by region and the combined analysis of all samples, showcasing the variation in cutotypes. C The proportion of different cutotypes observed, when age, moisture, and sebum are evenly grouped according to their distributions. D The relative abundance of the driver of the cutotypes, when age, moisture, and sebum are evenly grouped according to their distributions. E The tile map showing the Pearson correlation between collected meta info and skin physio-optical properties. The width of curves is the explained variance of these factors to metagenome structure calculated with Envfit test, and colors for its p-value. F Mediation analysis examining relationship between age and microbiome structure, considering significant influencing factors such as sebum, moisture, and sensitivity. The arrow from the independent variable (age) to the mediating variable (sebum, moisture, and Tivi) illustrates the influence of the independent variable on the mediating variable. Likewise, the arrow from the mediating variable to the dependent variable (microbiome structure) demonstrates the impact of the mediating variable on the dependent variable. Finally, the arrow from the independent variable to the dependent variable indicates the overall effect of the independent variable on the dependent variable, encompassing both total effect and average direct effect. Average direct effects (ADE) refers to the direct effects of the independent variable on the dependent variable. It represents the effect of the independent variable on the dependent variable while controlling for the mediating variable(s). Total effect, on the other hand, refers to the overall effect (direct + indirect) of the independent variable on the dependent variable. p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test
In comparison, we constructed cutotypes using the genus-level abundances annotated by metaPhlAn4 [34], one of the most widely used taxonomic profiling tools. However, it is important to note that metaPhlAn4 assigned a median of only 54% abundances to known genera, resulting in fewer annotated genera and lower α-diversity compared to FMGC in our samples (Fig. S6). When analyzing all samples combined, metaPhlAn4 generated two cutotypes: the Prevotella-cutotype and the Cutibacterium-cutotype. However, significant inconsistencies emerged when analyzing the samples separately by region, ~ 51% of samples’ cutotypes were discordant. Specifically, ~ 42% Prevotella-cutotype samples shift to Streptococcus-cutotype (~ 29% to Corynebacterium-cutotype; ~ 18.22% to Malikia-cutotype; ~ 9.81% to Porphyromonas-cutotype; ~ 0.93% to Cutibacterium-cutotype), while ~ 4.2% Cutibacterium-cutotype samples shift to Corynebacterium-cutotype (~ 2.9% to Porphyromonas-cutotype).
These findings highlight the importance of using FMGC as a better catalogue of reference genomes for further characterizing the facial microbiome, emphasizing the need for more complete and accurate reference genomes to obtain stable and reliable cutotype classifications for further investigations.
Age as a determinant of facial microbiome composition
With the availability of skin physio-optical features and participants’ metadata, we investigated the influence of these factors on facial microbiome. Remarkably, age, moisture, and sebum were identified as key factors that correlated with both cutotypes and driver taxa, exhibiting distinct trends (Fig. 2C). Specifically, we observed a notable decline in the proportion of the C-cutotype with increasing age and higher levels of skin moisture, while it exhibited a significant increase with elevated sebum levels (Fig. 2C). Conversely, the S-cutotype displayed an opposite pattern, indicating a contrasting response to these skin properties (Fig. 2C, S7). These findings were further supported by the relative abundance of the two driver genera, Streptococcus and Cutibacterium, which exhibited similar patterns to their respective cutotypes (Fig. 2D, S8). These results are consistent with previous findings [25, 30].
We further quantified the influence of the aforementioned factors on the facial microbiome using an Envfit test (“Methods” section). Consistent with the cutotype analysis, all of these factors demonstrated significant correlations with the metagenome structure (measured with axis 1, and 2 of the principal coordinate analysis (PCoA) of facial microbial profiles), with the exception of R2 (an elasticity measurement) and pH (Fig. 2E). Among them, age, moisture, sebum, and sensitivity emerged as the top four factors (Fig. 2E). Notably, significant inter-correlations were observed among these factors, with age demonstrating correlations with most of the other factors (Fig. 2E). Specifically, age displayed negative correlations with skin elasticity measures (R2, R5, and R7), sebum, and gloss, while demonstrating a positive correlation with Sensitivity (Tivi). To better understand the individual contributions of these factors, we conducted a mediation analysis [41,42,43], which revealed that age had the most substantial and direct impact on the community structure of the facial microbiome (measured with axis 1, 2, and 3 of PCoA, Fig. 2F). For example, age exerts a significant influence on both sebum levels and the structure of the microbiome, where the latter is influenced both directly and indirectly by age; when accounting for the mediator, age continues to exhibit a significant average direct effect, which is stronger than that of the sebum level to the facial microbiome (Fig. 2F).
Together, age stood out as the factor exhibiting the strongest correlation with the abundances of specific bacterial species in our exploration of the correlation between individual bacterial species and the physio-optical properties of the skin, as well as other meta-information (partial Pearson correlation, p < 0.05; Fig. S9). Considering that age is an independent characteristic not influenced by any other factor, while other physiological properties are correlated with age (e.g., the older the age, the less sebum), we used it as a primary variable in our mediation analysis. Following the same logic, we argue that the observation of a stronger age-microbe correlation may suggest a causal relationship from age to microbial composition.
FMGC reveals hundreds of novel age-related MAGs
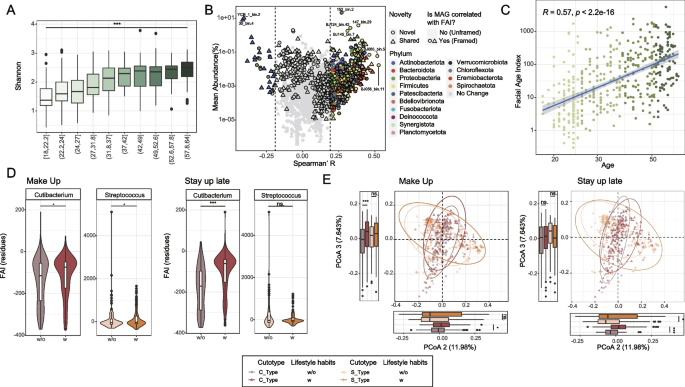
We next focused on the age-related effects in the facial microbiome. We found a significant increase in the α-diversity of the facial microbiome, as indicated by the Shannon index, with advancing age (Fig. 3A). This observation was supported by an overall Pearson correlation coefficient of 0.47 and a p value < 0.001 (Fig. S10), consistent with previous research [11, 16]. The overall trend was partially driven by the higher α-diversity of the S-cutotype comparing to the C-cutotype (Fig. S11) and the increased proportion of the S-cutotype in the older age groups (Fig. 2C). Furthermore, similar patterns, such as the increased α-diversity with advancing age, were consistently observed within individual cutotypes (Fig. S12). These trends may reflect the declining functionality of the facial immune system, resulting from continuous exposure to environmental and/or chemical factors[44]. Furthermore, they suggest a potential connection between aging, increased microbiome diversity and susceptibility to certain diseases.
A microbiome-based facial age index (FAI) reveals effects of suboptimal lifestyle habits on skin aging. A Barplot showing the increase in Shannon diversity with advancing age. B Scatter plot displaying MAGs correlated with the calculated Facial Aging Index (FAI). Labeled dots represent MAGs with mean abundance higher than 1% or relatively higher absolute Spearman’s R (≥ 0.5). Framed dots indicate MAGs that also exhibit strong correlations with FAI. C Facial age index (FAI) built with marker microbes and its significantly positive correlation with chronical age, confirming the practicability of using facial skin microbes to access skin age. Noted that Wuhan samples were not included in the construction of FAI due to its limited age range and inclusion of acne samples. D Boxplot showing the FAI-residuals (with the effects of the chronical age removed) in participants with/without certain lifestyle habits and different cutotypes. E PCoA plot demonstrating the difference of overall microbiome structure among participants with/without certain lifestyle habits and different cutotypes. p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test
To identify individual microbial genomes (MAGs) significantly correlating with age, we scrutinized the dataset, excluding 269 samples with potential confounding factors such as acne and make-up habits [45, 46]. The refined dataset of 229 samples revealed a total of 685 age-associated MAGs (p < 0.05, Pearson’s correlation; Fig. 3B, Table S3). Among these, 652 MAGs (338 novel MAGs) displayed positive correlations with age (pMAGs), while only 31 (21 novel MAGs) exhibited negative correlations (nMAGs). Interestingly, a closer inspection revealed that the Prevotella genus, known as a keystone genus in human gut while often found associated with human infection in other body sites [47], contained the highest number of pMAGs (47 out of 652; Fig. S13A), followed by Streptococcus and Neisseria (44 and 30, respectively), two of the known members of the oral microbiota. These two genera include several pMAGs capable of opportunistic pathogenicity, such as Streptococcus oralis [48] (5 MAGs, including 3 novel ones), Streptococcus mitis [49] (21 MAGs, 14 novel ones), Neisseria subflava [50] (5 MAGs, 1 novel). Surprisingly, there are 8 pMAGs (6 novel ones) belong to the notorious pathogen Streptococcus pseudopneumoniae [49]. On the other hand, Corynebacterium contains MAGs from different species correlated positively (27 MAGs) or negatively (12 MAGs) with age (Fig. S13A). For example, BJ042_bin.19 is a novel pMAG of Corynebacterium macginleyi, reported as a pathogen in case of unilateral conjunctivitis [51], that positively correlates with age showing a Spearman’s R of 0.42, while HC31_bin.3 is a Corynebacterium singular negatively correlates with age exhibiting a Spearman’s R of − 0.33(Table S3), suggesting heterogeneity exists within the Corynebacterium genus. Besides, the Cutibacterium contains the second highest number of negatively correlated MAGs (7 nMAGs), consistent with our above observations.
Focusing on MAGs with stronger correlations (absolute Spearman’s R ≥ 0.5) or with high mean relative abundance (≥ 1%) across samples (Fig. S13B), we identified a subset of eight MAGs, comprising six pMAGs and two nMAGs. Among which, two nMAGs, 35_bin.4 and YCR_1_bin.2, from Cutibacterium acnes stood out, with a mean relative abundance ranging from ~ 8 to 10% and high Spearman’s R, consistent with the known negative associations between C. acnes and aging [16]. Surprisingly, all of the six pMAGs are identified as novel MAGs, including four with no species-level assignment from four different genera (Leuconostoc, Enterobacter, Anaerococcus, and Streptococcus). Specifically, the two low-abundant yet strongly correlated pMAGs both belong to the Anaerococcus genus, including one from Anaerococcus nagyae, a species previously reported to be associated with fatal sepsis in patients undergoing transarterial chemoembolization treatment [52].
Experimental validation is imperative to elucidate the roles of these age-related MAGs, particularly the novel ones. Subsequent experiments should shed light on their functional significance.
A microbiome-based Facial Age Index reveals cutotype-dependent effects of lifestyles on skin aging
To quantitatively access the age-related effects to the facial microbiome, we further identified age-related taxa at higher taxonomic levels (Table S3) and used them together with the age-related MAGs to develop a Facial Aging Index (FAI). The FAI takes into account both variations in taxa abundance and the correlations between bacterial composition and age (“Methods” section). A higher FAI score indicates a greater degree of skin aging. To validate the effectiveness of our approach, we constructed the FAI using data from a specific region (e.g., Guangzhou) and then tested it on samples from different regions (e.g., Beijing). We assessed the correlation between chronological age and FAIs in the test dataset. Regardless of the region used to build the FAI, the test results remained consistent across other regions (Fig. S14). Applying this method to all our samples, we discovered a positive correlation between the FAI and the chronical age of the participants, both when combined (p < 2.2e-16, Pearson’s r = 0.55, Fig. 3C) and within individual cutotypes (C-cutotype: p < 2.2e-16, Pearson’s r = 0.54; S-cutotype: p = 2.3e-11, Pearson’s r = 0.47, Fig. S15). Specifically, 557 out of the 652 pMAGs were found to correlate with FAI, while all 31 of the nMAGs also exhibited correlations with FAI (Fig. 3B, S13C).
Interestingly, when examining the impact of different lifestyles on the facial microbiome, which are often related to skin conditions [46, 53, 54], we observed significantly higher FAI-residuals (controlled with age) in individuals with certain lifestyles, including “Make up” (excessive or heavy makeup application, defined as three or more cosmetic applications per week; see “Methods” section) and “Stay up late” (going to bed after 11 pm on three or more occasions per week, indicating habitual sleep restriction; see “Methods” section). After accounting for the participants’ chronological age, we found significantly elevated FAI residuals in the “Make up” group compared to the group without make-up in both the C– and S-cutotypes (Fig. 3E). We observed similar trends in the “Stay up late” group, although the difference was only statistically significant in the C-cutotype, not the S-cutotype. Notably, since the C-cutotype was enriched with young participants (Fig. 2C), our analysis suggests that the “Stay up late” lifestyle has a greater impact on the younger population than the older population. Furthermore, we observed relatively lower sebum-level-residuals (p < 0.1, Wilcoxon rank sum test), significantly lower R5-residuals (skin elasticity, the higher the better; p < 0.05, Wilcoxon rank sum test), and higher Tivi-residuals (sensitivity, the lower the better; p < 0.05, Wilcoxon rank sum test), in S-cutotype samples with unhealthy lifestyles (Fig. S16). Additionally, other host conditions such as constipation and “Mood Swings” (referring to frequently significant emotional fluctuations) were also found to significantly affect skin aging (Fig. S17). It is noteworthy that “Mood Swings” specifically impact participants with the C-cutotype (Fig. S16).
We further confirmed the effects of lifestyles on facial microbiome using PCoA analysis, which taking into account differences in all microorganisms. We again observed significant differences in the overall between group diversities in participants with and without “Make UP” and “Stay up late” lifestyles. As shown in Fig. 3E, we used the main axis 2 and 3 of the PCoA analysis to analyze the influence of lifestyles on facial microorganisms, because the main axis was mainly driven by the cutotype (Fig. S18). Our results showed that “Stay up late” significantly affected the microbial structure in both cutotypes, while “Make up” only affected the C-cutotype.
Specifically, we observed a higher relative abundance of the pMAGs in samples with makeup habits, while the nMAG were enriched in samples without makeup habits (Fig. S13D). Furthermore, we also assessed the total abundance of the pMAGs and the nMAGs, and the results remained consistent. This suggests a potential association between makeup habits and MAG-level changes in facial microbes.
Together, our findings suggest that age-associated microbial markers can serve as indicators to characterize skin age, and distinct cutotypes exhibit varied responses to external influences.
Cutotype-dependent aging effect in microbial metabolite pathways due to unhealthy lifestyles
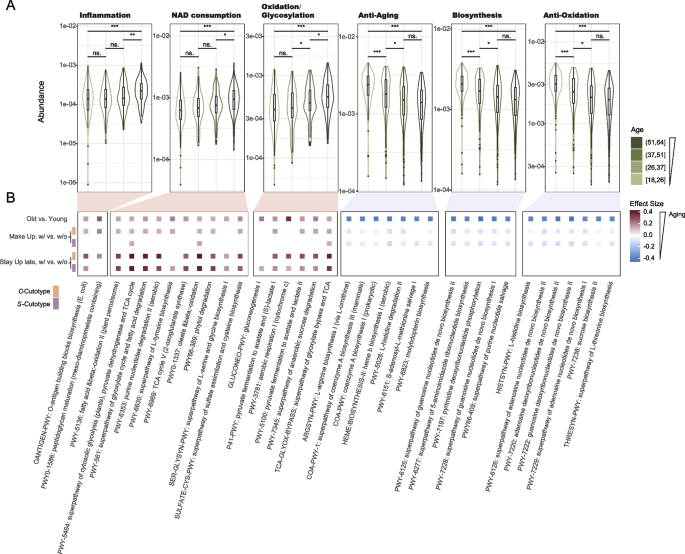
We next investigated the microbial functional changes related to facial skin aging. We annotated the microbial functions using HUMAnN3 [55]. These functions were then grouped into seven categories based on their MetaCyc annotations [56], namely Inflammation, Nicotinamide adenine dinucleotide (NAD) consumption, Oxidation/Glycosylation, Anti-aging, Anti-Oxidation, Biosynthesis, and Others. We then conducted a PERMANOVA analysis to determine the role of aging in the abundance structure of the microbial pathways, and discover that age is the only significant factor (Fig. S19A). To investigate age-related pathways, we classified the participants into four equal-sized groups based on their age: Group A (18–25 years), B (26–37 years), C (37–51 years), and D (51–64 years). Using LefSe analysis [57], we compared the youngest group (Group A) with the oldest group (Group D) and identified 61 and 237 pathways that were significantly more abundant in Group A and D, respectively (Table S4).
Notably, pathways associated with the first three categories were known to be associated with aging [58,59,60,61,62,63,64,65,66]. All pathways in these three groups showed significant enrichment in the Group D (Fig. 4). We collectively refer to these pathways as AG-pathways (Table S4). For instance, the OANTIGEN-PWY pathway, categorized under Inflammation, is responsible for producing O-antigen, a repeating unit of lipopolysaccharides (LPS) known to trigger inflammatory responses [67]. We observed a significantly enrichment of this pathway in oldest group (Fig. S20) with the highest linear discriminant analysis (LDA) score of 3.30. Another pathway, PWY-5136, falls under NAD consumption and employs NAD in the process of fatty acid β-oxidation. This pathway generates reactive oxygen species (ROS), leading to mitochondrial damage and oxidative stress [68,69,70]. The increasing prevalence of the NAD consumption pathway underscores its potential significance, explaining the inclusion of NAD in skincare products designed for the advanced age group. Additionally, besides the individual pathways, the cumulative abundance of pathways within each of the broad functional categories demonstrated an upward trend with advancing age (Fig. 4A).
Functional analysis reveals molecular pathways underlying skin aging and their associations with suboptimal lifestyle habits. A Boxplot showing the accumulated abundance of aging-related (Inflammation [58, 59], NAD consumption [60,61,62, 71], and Oxidation/Glycosylation [63,64,65,66]; red shadows) and anti-aging-related (Anti-Aging, Biosynthesis, and Anti-Oxidation; green shadows) pathways across different age groups. B Tile map plot displaying the effect size, as determined by the Wilcoxon rank sum test (filtered with adjusted one tailed p value < 0.1), between the youngest and oldest age groups, with or without make up habits and with or without stay-up-late habits. p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test
Conversely, pathways associated with Anti-Aging, Biosynthesis, and Anti-Oxidation are found to be enriched in Group A, thus referred to as anti-AG-pathways. For example, the ARGSYN-PWY (l-arginine biosynthesis pathway) of the Anti-Aging group generates l-arginine. Arginine is often used in anti-aging skincare products because of its potential ability to support collagen production [72, 73]. Meanwhile, the PWY-6126 (superpathway of adenosine nucleotides de novo biosynthesis II) of the Anti-Oxidation group is a metabolic pathway that participates in the de novo biosynthesis of adenosine nucleotides, which are involved in oxidative stress response, glutathione biosynthesis, and DNA synthesis under anaerobic conditions. Overall, the total abundance of the pathways within each group also displayed a decreasing trend with the advancing age (Fig. 4A). It is worth to mention that this grouping is based on sample size, and is merely for better presenting the result. Using an alternative grouping method according to skin wrinkles [74] generated essentially the same results (Fig. S21). In fact, when age was used as numerical values, we observed significant correlations between age and the pathway abundances in manners that are consistent with the grouped ages (Fig. S19B). In particular, the chronical age negatively correlated with Anti-Aging (R = − 0.31 and p = 1.7e-11), Biosynthesis (R = − 0.3 and p = 4.9e-11) and Anti-Oxidation (R = − 0.29 and p = 1.6e-10), and positively correlated with Inflammation (R = 0.2 and p = 2.4e-05), NAD consumption (R = 0.2 and p = 2.4e-05), and Oxidation/Glycosylation (R = 0.21 and p = 5.3e-06). These results indicate that our observations are robust again the age grouping methods.
We further investigated whether the impact of the above mentioned lifestyles on the facial microbiome is reflected in microbial functions. To explore this, we controlled for chronological age differences between groups and compared pathway abundances among groups with different lifestyles. Consistent with the FAI analysis, we observed significantly higher abundances of AG-pathways and lower abundances of anti-AG-pathways in the “Make up” group (Wilcoxon rank sum test; Fig. 4B). These trends were consistently observed in both the C– and S-cutotype groups. Interestingly, while the FAI analysis indicated that “Stay Up Late” primarily affected the C-cutotype group, our findings revealed that metabolite pathways in participants belonging to both cutotypes were influenced by this lifestyle. However, the influence of “Stay Up Late” on microbial functions were only statistically significant in the AG-pathways but not on the anti-AG-pathways (Fig. 4B).
Should these alterations in metabolic pathways be experimentally validated, they could elucidate the potential molecular mechanisms underlying skin aging and provide potential intervention targets to counteract the aging process. Our analysis further emphasizes the impact of specific lifestyles on aging and highlights the importance of personalized intervention strategies tailored to individual lifestyles. For example, exploring the supplementation of NAD and anti-aging metabolites may be considered as strategies to mitigate skin aging in older age groups or in individuals with habitual sleep restriction.



Add Comment