ATT treatment, as opposed to ART, significantly reduced the severity of liver inflammation and fibrosis induced by S. japonicum infection
Anti-inflammatory and antifibrotic effects have been observed in various ART analogs. Mice infected with S. japonicum for 6 weeks exhibited pronounced liver inflammation and fibrosis. Our findings reveal that 6 weeks of S. japonicum infection markedly increased the concentration of sera ALT and AST, both indicators of liver inflammation or damage [26]. Importantly, treatment with ATT, but not ART, significantly decreased these levels (Fig. 1b) (t-test: Sj(−)/NC versus Sj6w/NC: ALT: t(5) = 6.37, P = 0.012; AST: t(6) = 2.56, P = 0.021. Sj6w/NC versus Sj6w/ATT: ALT: t(7) = 1.15, P = 0.026; AST: t(8) = 2.41, P = 0.0064). Furthermore, S. japonicum eggs-induced liver granulomas, which can be classified into pre, early, mature, and late stages [6, 7], were substantially reduced by ATT treatment alone. This reduction was particularly notable in granulomas at their early and mature stages, as evidenced by H&E staining (Fig. 1c and d; ANOVA: F(8,73) = 2.27, P = 0.032. Sj6w/NC versus Sj6w/ATT: mature: P = 0.035; Sj6w/ART versus Sj6w/ATT: early: P = 0.001, mature: P = 0.0017).
The primary markers for activated hepatic stellate cells (HSCs) and myofibroblasts in mice are α-SMA and collagen secretion. Notably, ATT treatment significantly lowered the expression of α-SMA, as evidenced by WB analysis (Fig. 1e; t-test: Sj6w/NC versus Sj6w/ATT: t(4) = 2.63, P = 0.029). Additionally, ATT significantly alleviated the area of collagen deposition surrounding individual eggs, as demonstrated by Sirius red staining of liver sections (Fig. 1f; t-test: Sj6w/NC versus Sj6w/ATT: t(9) = 4.26, P = 0.0011). These findings suggest that ATT is more effective than ART in ameliorating liver fibrosis induced by S. japonicum infection in mice.
ATT demonstrated a potent schistosomicidal activity in mice, surpassing the effect of ART
ART and its derivatives are known to decrease the number of female adult worms or increase the count of single male worms, thereby reducing the total burden of adult worms. Hererin, treatment with either ATT or ART with a very low dose (5 mg/kg body weight) significantly reduced the total number of S. japonicum adult worms in the portal and mesenteric veins of mice with schistosomiasis japonica, compared with the untreated group (Fig. 2a; t-test: Sj6w/NC versus Sj6w/ATT: t(6) = 2.43, P = 0.026). Notably, the count of single male worms increased significantly (Fig. 2a; t-test: Sj6w/NC versus Sj6w/ATT: t(8) = 2.53, P = 0.018. Sj6w/NC versus Sj6w/ART: single male: t(7) = 2.33, P = 0.026). Notably, the count of single male worms increased significantly (Fig. 2a). Furthermore, within the liver, ATT treatment significantly reduced the number of deposited eggs, a result not observed with ART (Fig. 2b, c; t-test: Sj6w/NC versus Sj6w/ATT: t(8) = 2.07, P = 0.036).
Enhanced anti-S. japonicum activity of ATT compared with ART in mice. a The total number of S. japonicum adult worms and individual male worms present in the hepatoportal and mesenteric veins of mice with 6 weeks of S. japonicum infection, with either ART or ATT treatment (n = 4–6). Data were thoroughly counted and analyzed using a t-test. b Enumeration of eggs in liver section (2 mm) from mice subjected to 6 weeks of S. japonicum infection, with or without ART or ATT treatment, was conducted and analyzed by t-test. Representative liver section (2 mm or 400 μm) post- H&E staining from these groups are exhibited in c. d Scanning electron microscopy (SEM) was employed to examine the surface morphology of adult worms extracted from the livers of infected mice, treated with or without ATT. Detailed views include the tegument of adult worms’ mid-body without (a1) or with (a2) ATT treatment. ATT’s impact is evident in the altered appearance of regular prominent spines (b1 versus b2), crests (c1 versus c2) in the mid-body tegument and prominent spines (e1 versus e2) on the gynecophoral canal of adult males (d1 versus d2). e, f Volcano plots illustrate the differential expression of genes (DEGs) in S. japonicum female (e) and male (f) adult worms from mice with or without ATT treatment, as analyzed by RNA-seq analysis (FDR ≤ 0.001 and log2 ≥ 1). The X axis represents the log2 fold change, and the Y axis represents the log10 (adjusted P value). In these plots, red and green represent up- and downregulated genes, respectively, while gray dots denote genes without significant changes. The threshold was set at adjusted P value < 0.05 and |log2 fold change| > 1. g, h Significant Gene Ontology (GO) categories for DEGs in S. japonicum female (h) and male (i) adult worms from mice treated with or without ATT. The X axis denotes the log10 (P value), and the Y axis lists the GO term names
To further elucidate the schistosomicidal efficacy of ATT, adult female and male worms extracted from mice were examined using scanning electron microscopy (SEM). As shown in Fig. 2d, apparent alterations were observed in both the mid-body surface of adult females and males, as well as in the surface of the males’ gynecophoral canal. ATT treatment resulted in the loss of apically directed spines and rupture of the crests. Additionally, the tegument of the gynecophoral canal exhibited extensive damage characterized by structural disorganization, collapse, and the presence of hole-shaped erosions.
To further examine the possible molecular mechanism of ATT’s anti-worm effect, DEGs in adult female and male worms, isolated from S. japonicum-infected mice with or without ATT treatment, were analyzed through RNA-seq. Each sample yielded approximately 20 million total sequence reads. RNA-seq analysis revealed significant changes in the transcription of 98 genes in females (Fig. 2e) and 48 genes in males (Fig. 2f) due to ATT treatment. Gene Ontology (GO) enrichment analysis highlighted that GO terms such as “cellular anatomical entity,” “catalytic activity,” “binding,” and “cellular process” were significantly represented among these altered genes. Notably, the majority of genes were predominantly associated with “cellular anatomical entity” (Fig. 2g, h).
These results suggest that ATT directly damages the surface structure of S. japonicum, particularly the surface of males’ gynecophoral canal, contributing to the reduction of female worms (or the increase in single male worms) and, subsequently egg reduction. This is likely due to transcriptional changes in genes associated with cellular anatomical entities.
Comprehensive analysis using both transcriptome and 23 cytokines’ assay of mouse liver lysates underscored the immune-modulatory effects of ATT
To explore the mechanisms underlying ATT’s influence on liver inflammation and fibrosis caused by 6 weeks of S. japonicum infection, RNA-seq and Bio-Plex Pro-Mouse Group I Cytokine 23-plex test were employed. RNA-seq revealed that ATT treatment significantly upregulated 38 genes and downregulated 148 genes in the liver tissues of infected mice (Fig. 3a). The Kyoto Encyclopedia of Gene and Genome (KEGG) pathway analysis identified that most of the genes related to immunomodulatory activities were enriched, including pathways like antigen processing and presentation, viral protein interaction with cytokines, inflammatory bowel disease, NOD-like receptor signaling, and Th1 and Th2 cell differentiation pathways (Fig. 3b).
RNA-seq and multi-cytokine analysis indicated the immunomodulatory effects of ATT on the liver of mice with 6 weeks of S. japonicum infection. a Volcano plot illustrating the DEGs in S. japonicum-infected mice liver, with or without ATT treatment. The X axis represents the log2 fold change, while the Y axis shows the log10 (adjusted P value). In this plot, red and green symbols highlight upregulated and downregulated genes, respectively, while gray symbols indicate genes with no significant difference. The threshold for significance is a set at an adjusted P value < 0.05 and |log2 fold change| > 1; b Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis identifies significant pathways affected by DEGs in the infected mice liver, with or without ATT treatment. The X axis indicates −log10 (P value), and the Y axis lists the names of affected pathways. c–e Concentrations of 23 cytokines were measured using Bio-Plex Pro-Mouse Group I Cytokine 23-plex assay. These cytokines include: pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) (c), colony-stimulating cytokines or chemokines (IL-3, G-CSF, GM-CSF, KC, MCP-1, MIP-1α, MIP-1β, RANTES, Eotaxin) (d), and activatory and differentiating cytokines for innate lymphoid cells or T cell subsets (IL-2, IL-12 (p40), IL-12 (p70), IFN-γ, IL-4, IL-5, IL-10, IL-13, IL-9 and IL-17A (e). Data represent mean ± SD from different experimental groups and were analyzed by t-test (n = 4–6). Significant differences are indicated by *P < 0.05 and **P < 0.01
The Bio-Plex Pro-Mouse Group I Cytokine 23-plex assay displayed significant changes in level of 18 cytokines and chemokines following 6 weeks of S. japonicum infection. There was a notable increase in proinflammatory cytokines such as IL-1α, IL-1β, IL-6, TNF-α (Fig. 3c; t-test: Sj(−)/NC versus Sj6w/NC: IL-1α: t(7) = 2.94, P = 0.011; IL-1β: t(7) = 4.60, P = 0.0012; IL-6: t(7) = 4.73, P = 0.0011; TNF-α: t(6) = 3.09, P = 0.0019), as well as colony-stimulating cytokines or chemokines such as G-CSF, KC, MCP-1, MIP-1α, MIP-1β, RANTES and Eotaxin (Fig. 3d; t-test: Sj(−)/NC versus Sj6w/NC: IL-3: t(6) = 3.349, P = 0.0077; G-CSF: t(6) = 5.49, P = 0.0008; GM-CSF: t(6) = 2.22, P = 0.034; KC: t(6) = 6.05, P = 0.0005; MCP-1: t(7) = 3.89, P = 0.0030; MIP-1α: t(6) = 2.47, P = 0.024; MIP-1β: t(6) = 4.881, P = 0.0014; RANTES: t(6) = 4.85, P = 0.0014; Eotaxin: t(7) = 4.05, P = 0.0024). Additionally, activator or differential cytokines for innate lymphoid cells or T cell subsets such as IL-12(p40), IFN-γ, IL-4, and IL-5 were significantly increased (Fig. 3e). Conversely, IL-3, GM-CSF and IL-17A levels were notably decreased. No significant changes were observed in IL-2, IL-10, IL-13, and IL-9 (Fig. 3e; t-test: Sj(−)/NC versus Sj6w/NC: IL-12(P40): t(6) = 2.003, P = 0.046; IFN-γ: t(6) = 1.98, P = 0.047; IL-4: t(7) = 4.99, P = 0.0008; IL-5: t(7) = 2.53, P = 0.019; IL-17A: t(6) = 1.98, P = 0.047). Post ATT treatment, there was a significant increase in G-CSF, MIP-1α, MIP-1β, RANTES, eotaxin (Fig. 3d; t-test: Sj6w/NC versus Sj6w/ATT: G-CSF: t(6) = 2.45, P = 0.025; MIP-1α: t(7) = 2.13, P = 0.035; MIP-1β: t(7) = 2.89, P = 0.012; RANTES: t(7) = 2.31, P = 0.027; eotaxin: t(8) = 5.97, P = 0.0002) and IL-12(p70), IL-4, IL-5, IL-10, and IL-9 (Fig. 3e; t-test: Sj6w/NC versus Sj6w/ATT: IL-12(P70): t(7) = 1.99, P = 0.043; IL-4: t(8) = 4.56, P = 0.0009; IL-5: t(8) = 3.91, P = 0.0022; IL-10: t(7) = 3.44, P = 0.0054; IL-9: t(8) = 2.03, P = 0.038) in the livers of infected mice. However, levels of IL-1α, IL-1β, IL-6, TNF-α (Fig. 3c), IL-3, GM-CSF, KC (Fig. 3d), IL-12(p40), IFN-γ, IL-13, and IL-17A (Fig. 3e) remained unchanged. These results suggest that ATT has significant immunomodulatory effects, especially in enhancing type 2 immune responses.
Effect of ATT or ART treatment on the count of immune cells in the liver or spleen of mice with 6w of S. japonicum infection
Artemisinin analogs have demonstrated context-dependent immunomodulatory effects in various cell types and diseases, influencing both innate and adaptive immune responses. To explore the influence of ATT on immune cell populations in the liver and spleen of mice infected with S. japonicum for 6 weeks, mononuclear cells were isolated and analyzed using respective markers (Figs. 4a, 5a).
Neutrophil, eosinophil and macrophage redistribution in the liver or spleen of S. japonicum-infected mice following ART or ATT treatment. Flow cytometry was used for these cells analysis. Nucleated cells were initially gated on the basis of size and complexity, as indicated by forward scatter area (FSC-A) and side scatter area (SSC-A). Subsequently, single cells were distinguished from doublets using a combination of FSC-A and FSC-H. a Within this single cell population, live immune cells were identified as CD45+. Neutrophils (Neu) were further defined using CD45+CD11b+ Ly6G+ markers, and eosinophils (Eos) were gated as CD45+CD11b+SiglecF+. Macrophages (MA) were identified as CD45+Ly6G−F4/80+. These MA were further classified into M0 (CD206−iNOS−), M1 (iNOS+), and M2 (CD206+) subpopulations. b The proportion of neutrophils within CD45+ cell population in both liver and spleen of infected mice, with or without ART or ATT treatment, was evaluated and analyzed by two-way ANOVA (n = 4–6). c The proportion of eosinophils among CD45+ cells in both the liver and spleen of infected mice, treated with or without ART or ATT, was evaluated and analyzed by two-way ANOVA. d, e The proportions of total macrophages and their M0, M1, and M2 subpopulations in the CD45+Ly6G− liver cells of infected mice, treated with or without ART or ATT, were evaluated and analyzed by one-way or two-way ANOVA, respectively. f The M1/M2 ratio (index) was presented and analyzed by one-way ANOVA. Data represent mean ± SD from different experimental groups. Significant differences are denoted by *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001
Altered Th1/Th2 index in the livers of infected mice treated with ATT compared with ART. a Flow cytometry was used to assess the proportion of Th1 and Th2 cells in the liver of indicated mice. The gating strategy involved identifying CD45+CD3+ cells, then subdividing them into CD45+CD3+CD4+IFN-γ+ (Th1) and IL-4+ (Th2) populations. b, c The amount of Th1 and Th2 cells in the liver of infected mice, with or without ART or ATT treatment, were evaluated. a and b display the typical cell populations for Th1 and Th2, and panel (b) providing their quantification, which was analyzed by two-way ANOVA. d The Th1/Th2 ratio (index) was analyzed by t-test. Data represent mean ± SD from different experimental groups. Significant differences are indicated by *P < 0.05 and **P < 0.01
After a 6-week period of S. japonicum infection, there was a notably increase in the percentage of neutrophils in the liver and spleen. The increase in the liver was significantly reversed following treatment with ATT and ART. However, both ATT and ART significantly increased these levels in the spleen, with ART showing a particularly strong effect (Fig. 4b; ANOVA: F(5,43) = 11.31. Sj(−)/NC versus Sj6w/NC: liver: P < 0.0001; spleen: P = 0.035. Sj6w/NC versus Sj6w/ART: liver: P = 0.0011; spleen: P < 0.0001. Sj6w/NC versus Sj6w/ATT: liver: P = 0.0003; Sj6w/ART versus Sj6w/ATT: spleen: P = 0.0067). Regarding eosinophils, S. japonicum infection significantly increased their percentages. Further, both ATT and ART treatments significantly increased the frequency of eosinophils in the liver and spleen (Fig. 4c; ANOVA: F(5,46) = 40.06. Sj(−)/NC versus Sj6w/NC: liver: P < 0.0001; spleen: P = 0.046. Sj6w/NC versus Sj6w/ART: liver: P < 0.0001; spleen: P = 0.0045. Sj6w/NC versus Sj6w/ATT: liver: P < 0.0001; spleen: P = 0.00019). S. japonicum infection also significantly increased the number of macrophages in the liver. This increase was further accentuated but not significantly with the administration of either ATT or ART (Fig. 4d; ANOVA: F(5,24) = 118.8. Sj(−)/NC versus Sj6w/NC: P < 0.0001). The amount of M0 macrophages significantly increased, while the count of M2 macrophages substantially decreased with both ATT and ART treatments. The count of M1 macrophages was not significantly affected by either ART or ATT (Fig. 4e; ANOVA: F (4,45) = 22.75. Sj6w/NC versus Sj6w/ART: M0: P < 0.0001; M2: P < 0.0001. Sj6w/NC versus Sj6w/ATT: M0: P < 0.0001; M2: P = 0.0012). Notably, compared with ART, ATT therapy markedly lowered the M1/M2 index levels (Fig. 4f; ANOVA: F(2,15) = 4.68. Sj6w/ART versus Sj6w/ATT: P = 0.026).
Furthermore, 6 weeks of S. japonicum infection significantly increased the amount of Th1 cells in the liver but did not affect Th2 cells. Neither ATT nor ART treatment significantly altered the count of Th1 and Th2 cells (Fig. 5b, c; ANOVA: F(5,48) = 11.33, P < 0.0001. Th1: P = 0.0032). However, ATT resulted in a significant decrease in the Th1/Th2 index (Fig. 5d; t-test: Sj6w/NC versus Sj6w/ATT: t(10) = 1.80, P = 0.040).
ATT-regulated host immunity was more strongly correlated with the extent of liver fibrosis and the count of single males compared with ART
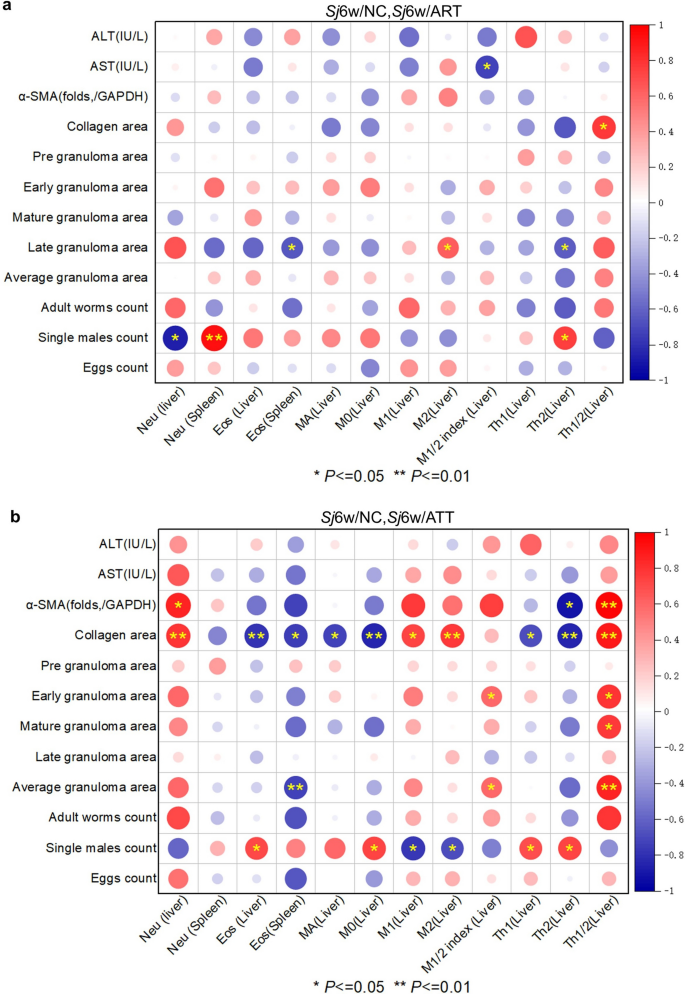
Correlation analysis highlighted more stars in 6 weeks of S. japonicum--infected mice with ATT treatment than ART treatment (Fig. 6a, b). During ART treatment, significant negative correlations were observed between liver neutrophil frequency and single male counts, spleen eosinophil frequency and liver Th2 frequency with the area of late granulomas, and the M1/M2 index in the liver with serum AST concentration. Conversely, significant positive correlations were noted between spleen neutrophils frequency and liver Th2 frequency with single male counts, liver M2 frequency with the area of late granulomas, and the Th1/Th2 index with collagen area (Fig. 6a; correlation: AST and M1/2 index: r(10) = −0.53, P = 0.017; collagen area and Th1/2: r(10) = 0.58, P = 0.028; late granuloma area and Eos (Spleen): r(11) = −0.44, P = 0.038; late granuloma area and M2 (Liver): r(11) = 0.40, P = 0.048; late granuloma area and Th2 (Liver): r(11) = −0.41, P = 0.047; single males count and Neu (Liver): r(9) = −0.75, P = 0.012; single males count and Neu (spleen): r(9) = 0.88, P = 0.0018; single males count and Th2 (Liver): r(9) = 0.56, P = 0.021). During ATT treatment, significant negative correlations were observed between the frequency of Th2 in the liver and α-SMA folds, the frequency of Eos in the liver or spleen, and various immune cells, including MA, Mo, Th1, or Th2, with the collagen area. Additionally, a negative correlation was found between the frequency of Eos in the spleen and the average granuloma area, and between the frequency of M1 or M2 in the liver and the count of single males. Conversely, significant positive correlations were noted between the frequency of Neu, Th1/Th2 index in the liver and α-SMA folds or collagen area, the frequency of M1 or M2 with the collagen area, the M1/M2 or Th1/Th2 index in the liver with early or average granuloma areas, and the Th1/Th2 index with the area of mature granuloma. Additionally, positive correlations were found between the frequency of Eos, M0, Th1, or Th2 in the liver and the count of single males (Fig. 6b; correlation: α-SMA and Neu (Liver): r(6) = 0.73, P = 0.03; α-SMA and Th2 (Liver): r(6) = −0.83, P = 0.012; α-SMA and Th1/2: r(6) = 0.94, P = 0.0057; collagen area and Neu (liver): r(11) = 0.63, P = 0.0061; collagen area and Eos (liver): r(11) = −0.62, P = 0.0066; collagen area and Eos (spleen): r(11) = −0.56, P = 0.013; collagen area and MA (liver): r(11) = −0.54, P = 0.015; collagen area and M0 (liver): r(11) = −0.71, P = 0.0011; collagen area and M1 (liver): r(11) = 0.53, P = 0.017; collagen area and M2 (liver): r(11) = 0.59, P = 0.0094; collagen area and Th1 (liver): r(11) = −0.47, P = 0.028; collagen area and Th2 (liver): r(11) = −0.72, P = 0.0019; collagen area and Th1/2 (liver): r(11) = 0.78, P = 0.0034; early granuloma area and M1/2 index: r(12) = 0.34, P = 0.046; early granuloma area and Th1/2: r(12) = 0.61, P = 0.013; mature granuloma area and Th1/2: r(12) = 0.59, P = 0.015; average granuloma area and Eos (spleen): r(12) = −0.54, P = 0.0098; average granuloma area and M1/2 index: r(12) = 0.34, P = 0.045; average granuloma area and Th1/2: r(12) = 0.72, P = 0.0039; single males count and Eos (liver): r(10) = 0.49, P = 0.034; single males count and M0 (liver): r(10) = 0.54, P = 0.015; single males count and M1 (liver): r(10) = −0.61, P = 0.013; single males count and M2 (liver): r(10) = −0.47, P = 0.041; single males count and Th1 (Liver): r(10) = 0.49, P = 0.037; single males count and Th2 (liver): r(10) = 0.54, P = 0.015). ATT-regulated host immunity showed a more significant correlation with the extent of liver fibrosis and the count of single males compared with ART, suggesting that ATT is more effective than ART in treating murine schistosomiasis japonica.
Correlation analysis of the frequency of host immune cells in the liver or spleen with the extent of liver inflammation or fibrosis, the size of granuloma area and the count of parasites. a, b Correlation plot of the frequency of neutrophils, eosinophils in the liver or spleen, the frequency of macrophages or their subsets M0, M1 or M2, M1/M2 index, and the frequency of Th1, Th2 and Th1/Th2 index in the liver with ALT and AST concentration, α-SMA expression folds (/GAPDH), area of collagen, pregranuloma, early granuloma, mature granuloma, late granuloma, and average granuloma, and count of worms, single males, and eggs from S. japonica at 6 weeks with or without ART (a) or ATT (b) treatment (Sj6w/NC, Sj6w/ART or Sj6W/ATT, n = 5–6) through OriginPro 2021. Significant correlation are indicated by *P < 0.05 and **P < 0.01








Add Comment