Synthesis and characterization of the dimeric prodrug F2A
We have previously reported an aromatic thioketal (ATK), which can be used as ROS-responsive linker to prepare multiple prodrugs [31, 33] or carriers [32]. In consideration of the carboxyl and hydroxyl groups of ATK and FA, respectively, the dimeric prodrug F2A was prepared by CDI-mediated esterification. As shown in Fig. 2A, CDI activates the two carboxyl groups of ATK to form acyl imidazole derivative ATK-CDI. The successful synthesis of ATK-CDI was confirmed by 1H NMR (Figure S1) and ESI–MS spectrum (Figure S2). In the second step, the intermediate ATK-CDI undergoes a substitution reaction with FA to obtain the final product, named F2A.
To demonstrate the exact chemical structure of F2A, multiple characterization methods were employed. The UV–VIS spectrum shows that compared to ATK, both FA and F2A exhibit stronger absorption at around 260 nm (Fig. 2B). This is caused by the double bond in the base group of FA, which serves as a nucleic acid analogue. This proves that FA and ATK are coupled. In order to more intuitively examine whether F2A is a dimeric prodrug, we used ESI mass spectrometry. Figure 2C shows the ESI–MS spectrum of F2A. ESI–MS m/z: calculated for F2A (C41H42F2N10O10S2) [M+H]+ 937.964, found 938.29; calculated for fragmentary peak of F2A (C27H25FN5O5S+) 550.585, found 550.05. These data were consistent with theoretical calculation. Moreover, it can be seen from the results that all the 1H NMR peaks can be assigned to F2A, and the ratio of protons represented by each peak is also consistent with the chemical structure of F2A (Fig. 2D). The above characterization results all prove that the dimeric prodrug F2A has been successfully prepared.
The ROS-responsive hydrolysis process of F2A
According to our previous reports related to ATK [31, 33], we proposed the possible hydrolysis process and products of F2A in ROS-containing media (Fig. 3A).
To verify this hypothesis, HPLC was used to verify the stability and response performance of the F2A (Fig. 3B, C and Figure S3). As shown in Fig. 3B, only one substance F2A was detected in PBS (pH 7.4) regardless of incubation for 12 h, 24 h or 72 h, which proved the excellent stability of F2A. However, additional substances such as FA, 9-fluorenone and γ-thiobutyrolactone are detected after incubation in 1 mM H2O2 for only 15 min. Moreover, with the extension of incubation time, the detected concentration of F2A gradually decreases while the concentration of other products gradually increases (Fig. 3C). After incubation for 1 h, the peak of F2A basically disappeared, indicating that F2A was completely degraded into other products, including the prototype drug FA. These results support our conjecture that F2A has H2O2-responsive drug release properties.
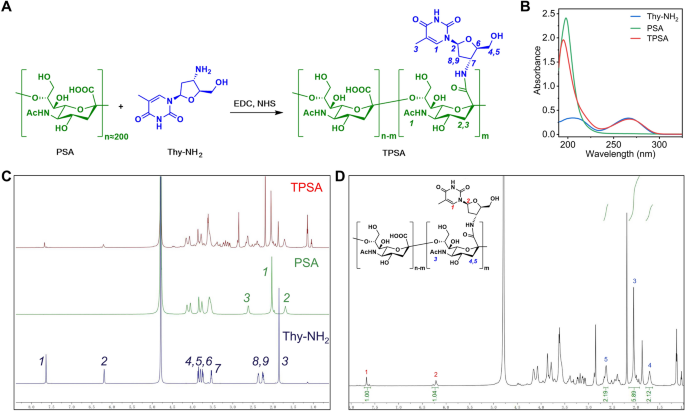
Synthesis and characterization of TPSA
Aiming to introduce massive thymine structures into PSA, Thy-NH2 was selected as thymine donor. TPSA was efficiently prepared by coupling Thy-NH2 with PSA in aqueous solution through EDCI/NHS mediated amidation reaction (Fig. 4A).
It can be seen from Fig. 4B that the UV–vis spectrum of PSA presents a single peak with λmax of 200 nm, while TPSA is slightly blue shifted to about 190 nm. Thy-NH2 has a relatively broad absorption peak around 210 nm and 260 nm respectively, similar to Fig. 2B, which is consistent with its characteristics as a nucleoside analogue. As expected, the TPSA sample also exhibits an absorption peak at 260 nm, indicating the presence of Thy-NH2 groups in TPSA.
As a polysaccharide, the difference between TPSA and PSA can be detected by 1H NMR. Specifically, 1H NMR can reflect more detailed proton information. Figure 4C shows the comparison of 1H NMR spectra of PSA, Thy-NH2 and TPSA. Excitingly, all the characteristic peaks of PSA and Thy-NH2 can be found in the spectrum of TPSA and are accurately assigned to each proton. It was confirmed that Thy-NH2 was successfully grafted onto PSA. Furthermore, by performing integral analysis on the peak area of the 1H NMR spectrum of TPSA (Fig. 4D), it can be concluded that there are about 50 Thy-NH2 in every 100 SA units, that is, the grafting rate of Thy-NH2 in TPSA is about 50%.
It is worth noting that the grafting rate of Thy-NH2 increases with the increase of feed ratio, reaching up to about 50% (data not shown). In order to ensure that the thymidine structure can form sufficient “Watson–Crick” base pairing (A = T)-like hydrogen bond with more F2A, and induce strong cross-linking between TPSA, the TPSA molecule with the highest grafting rate (50%) was selected for subsequent experiments.
Preparation, characterization and formation mechanism of supramolecular nanoprodrug (F@TPSA)
According to our hypothesis, the adenine structure of the dimeric prodrug can produce hydrogen bonds with the thymine structure of TPSA. In brief, simulating the process of “high temperature denaturation and low temperature annealing” can promote the formation of hydrogen bonds. However, since the highly complex 1H NMR spectra of TPSA are almost impossible to be fully identified, Molecular dynamics simulation and mass spectrometry were used to verify the hydrogen bond between FA and Thy-NH2.
Figure 5A shows the molecular dynamics simulation results of the interaction between FA and Thy-NH2. It can be seen that there are two hydrogen bonds similar to “A = T” between FA and Thy-NH2, with distances of 3.39 Å and 3.94 Å respectively. In Fig. 5B, a new peak at m/z 527.1304 appeared in the mass spectrum of mixed solution of FA and Thy-NH2 (molar ratio = 1:1) in PBS (pH 7.4), while completely disappeared at pH 5.0. This result indicated the formed hydrogen bonds would keep relatively stable under neutral, while be effectively broken under acidic environment (pH 5.0).
Inspired by Watson–Crick base pairing, supramolecular nanoprodrug F@TPSA was prepared by non-covalent cross-linking between dimeric prodrug F2A and functional material TPSA. As shown in the digital photographs and TEM images of Fig. 6A, F@TPSA was dispersed in PBS (pH 7.4) solution to form a colloidal system with obvious Tyndall effect and regular sphericity observed, while the PBS solution of TPSA was transparent, and there was no Tyndall effect (Figure S4). Although the particle size distribution of F@TPSA measured by DLS in aqueous solution is relatively broad, it still maintains a unimodal distribution trend (Fig. 6B), and the average hydrodynamic size is about 166.6 ± 3.9 nm (Fig. 6C), which is also consistent with TEM results. Notably, F@TPSA lost the Tyndall effect when dispersed in 1 mM H2O2 or PBS (pH 5.0) media. The irregular sphericity was observed by TEM, while the multi-modal particle size distribution was measured by DLS. The average hydrodynamic size also decreased to 110.1 ± 2.6 nm and 61.0 ± 2.5 nm, respectively. In contrast, it seems that PBS (pH 9.0) does not cause changes in mean particle size, and particle size distribution. This suggests that F@TPSA can remain stable under physiological or alkaline conditions, while structural damage occurs in acidic or ROS environments, entirely due to the properties of hydrogen bonding and ROS-responsiveness of ATK. Meanwhile, in Fig. 6D, TPSA as the shell, provides a significant negative ζ-potential for F@TPSA, which guarantees the biocompatibility of F@TPSA in vivo. The drug loading content of F@TPSA is 32.5%.
Characterization of supramolecular nanoprodrug F@TPSA. A The digital photos and TEM photographs of F@TPSA. B The particle size distribution of F@TPSA in different media. C Comparison of the average hydrodynamic size of F@TPSA in different media. D The ζ-potential of F@TPSA in PBS with pH 7.4 or pH 9.0. All data are presented the mean ± SD (n = 6)
From the above results, it can be inferred that hydrogen bonding forces dominate the formation of supramolecular nanoprodrug F@TPSA. The two terminal adenine structures of F2A can effectively form hydrogen bonds with a large number of thymine structures in TPSA and induce tight cross-linking between TPSAs. This supramolecular aggregate with appropriate hydrophobicity–hydrophilicity balance can further self-assemble into compact nanoparticles. The hydrophobic F2A is located in the core, while the TPSA chain serves as a hydrophilic shell. F@TPSA has a unique drug loading method and strong cross-linking enhanced stability, which can improve the water solubility and stability of F2A. Furthermore, due to the characteristics of hydrogen bonding and the ROS responsiveness of ATK, F@TPSA can achieve structural dissociation and drug release in acidic or oxidative stress environments. This provides a foundation for the subsequent application of F@TPSA.
F@TPSA specifically targets CD22+ B lymphoma cell lines
The above results all prove that the supramolecular nanoprodrug F@TPSA has been successfully prepared. Next, it is necessary to confirm whether F@TPSA can be specifically uptake by CD22 positive B lymphoma cell lines. Since F@TPSA has no fluorescent properties, F@TPSA needs to be labeled. Therefore, the hydrophobic small molecule Cy5 NHS ester can be embedded with the formation of F@TPSA and is named Cy5@F@TPSA. Similarly, Cy5@F@PLGA (note: not containing PSA) was prepared as a control. Two B lymphoma cell lines (Raji cells and Ramos cells) and two other lymphoid cell lines (RAW264.7 cells and THP-1 cells) were stained using FITC-labeled CD22 antibodies. The CD22 expression levels of two B lymphoma cell lines were significantly higher than those of the other two monocyte lines measured by flow cytometry (Figure S5). Especially, the average expression level of CD22 in Raji cells is the highest, 30.3 times that of RAW264.7 cells, indicating a significant difference in CD22 expression levels between the two cell lines. Therefore, Raji cells and RAW264.7 cells were selected as the study subjects for comparison of cellular uptake.
Figure 7A shows the flow cytometry of Raji cells or RAW264.7 cells after co-incubation with Cy5@F@TPSA. However, as can be seen from Fig. 7B, the proportion of Cy5@F@TPSA positive Raji cells reached 80.26 ± 1.55%, which was significantly different from 45.11 ± 1.32% positive rate in RAW264.7 cells. This suggests that F@TPSA is more likely to be taken up by B lymphoma cell lines than RAW264.7, thereby forming specific targeting of CD22+ cells.
Specific uptake of Cy5-labeled supramolecular prodrug of fludarabine (Cy5@F@TPSA) by B lymphoma cells in vitro. A, B Flow cytometry (A) and statistical analysis (B) of the endocytosis of Cy5@F@TPSA in Raji cells or RAW264.7 cells. C Comparison of endocytosis of two Cy5 labeled nanodrug (Cy5@F@TPSA and Cy5@F@PLGA) in CD22− RAW264.7 cells. D, E Flow cytometry (D) and intracellular Cy5 relative fluorescence intensity (RFI) of CD22+ Raji cells (E). Cy5@F@PLGA was used as a positive control and αCD22 was a neutralizing antibody to CD22. F, G Flow cytometry (F) and statistics (G) of Cy5@F@TPSA at different concentrations (0.25, 0.5, 1, 2, 4, 8 μg/mL) after phagocytosis by Raji cells. For E, n = 6, ∗∗∗p < 0.001
To further demonstrate the cell selectivity of F@TPSA, the uptake levels of Cy5@F@TPSA and Cy5@F@PLGA in RAW264.7 cells was compared (Fig. 7C and Figure S6). Obviously, there was no difference in the uptake of Cy5@F@TPSA and Cy5@F@PLGA by RAW264.7 cells, which ruled out the possibility that CD22-negative cells had an uptake preference for F@TPSA. Remarkably, the uptake of Cy5@F@TPSA by Raji cells was about 2.3 times that of Cy5@F@PLGA (Fig. 7D, E). Dramatically, the uptake of Cy5@F@TPSA by Raji cells was significantly inhibited after pre-incubation of CD22-neutralizing antibody (αCD22). This further confirms that the specific targeting of F@TPSA to B lymphoma cell lines is mediated by CD22, which is also consistent with previous reports [29]. Furthermore, from Fig. 7F, G, it can be observed that under the same incubation time condition, Cy5 fluorescence intensity in Raji cells gradually increases with the increase of F@TPSA concentration, indicating that CD22-mediated endocytosis is concentration-dependent.
In vitro tumor inhibition effects of F@TPSA in different cell lines
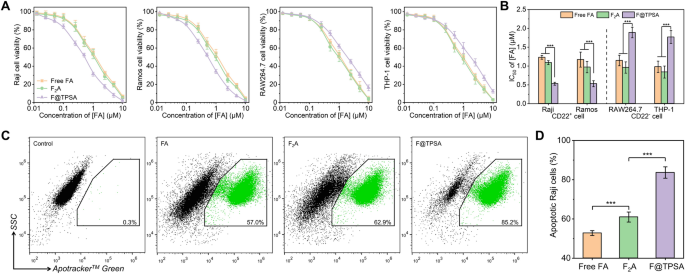
After demonstrating the significant advantage of F@TPSA in specifically targeting CD22 positive B lymphoma cells, it is reasonable to speculate that F@TPSA has stronger cytotoxicity towards the aforementioned cells. The MTT method can be used to test the effects of different concentrations of FA preparations on the cell viability of four types of cells (including B lymphoma cells and other cells). As shown in Fig. 8A, as the concentration increases, all cell viability exhibits a non-linear decline. The apoptosis rates of Raji, Ramos, RAW264.7 and THP-1 cells were all over 80% at high concentrations (10 μM FA), regardless of FA, F2A or F@TPSA. However, below 10 μM concentration, F@TPSA had a stronger negative effect on the viability of Raji and Ramos cells than FA and F2A. Accordingly, it can also be seen from Fig. 8B that the IC50 of FA and F2A in Raji cells are 1.2 ± 0.1 μM and 1.1 ± 0.1 μM, respectively. Surprisingly, the IC50 for F@TPSA is significantly reduced to 0.5 ± 0.1 μM. Similarly, when the IC50 of FA or F2A to Ramos cells is 1.2 ± 0.2 μM and 1.0 ± 0.2 μM, respectively, Ramos cells also exhibit a more sensitive state to F@TPSA (the IC50 is 0.5 ± 0.1 μM). More interestingly, F@TPSA showed higher IC50 than FA and F2A in two types of CD22 negative cells (RAW264.7 cells and THP-1 cells). It may be due to the lack of CD22 expression in RAW264.7 cells and THP-1 cells, resulting in weak uptake of F@TPSA, or due to low ROS levels in non B lymphoma cells, making it difficult to release FA encapsulated by TPSA. This also proves from another perspective that F@TPSA, a supramolecular nanoprodrug, not only enhances the cytotoxicity of FA to B lymphoma cell lines, but also enhances the tolerance of FA to other CD22-negative cells.
In vitro anti-tumor effects of Free FA, F2A, and F@TPSA in different cell lines. A Cytotoxicity of three FA formulations (Free FA, F2A, and F@TPSA) against CD22-positive B lymphoma cell lines (Raji cells, Ramos cells) or CD22-negative other lymphocyte lines (RAW264.7 cells, THP-1 cells) was measured by MTT assay. B The half inhibitory concentrations (IC50) of the three FA formulations on the above cells were compared. C, D Representative flow cytometry plots (C) and data statistics (D) of Raji cells treated for 1 h with three different FA formulations (all calculated as 1 μM FA). All data are presented the mean ± SD (n = 6). ***p < 0.001
In addition, the apoptosis effects of three FA preparations (FA, F2A, F@TPSA) on Raji cells were further observed by flow cytometry (Fig. 8C). Apotracker™ Green, as a fluorescent probe, can label apoptotic cells by detecting phosphatidylserine residues located outside the cell membrane. As can be seen from Fig. 8D, although FA and F2A (1.5 μM [FA]) could induce 52.8 ± 1.2% and 61.0 ± 2.6% apoptosis of Raji cells, respectively, F@TPSA caused led to more Raji cell apoptosis (83.7 ± 2.9%) under the same concentration. It is worth noting that TPSA has no effect on the cell viability of Raji cells up to 100 μM (Figure S7). Therefore, the stronger apoptotic efficiency exhibited by F@TPSA can be attributed to the more precise delivery of FA by TPSA.
Pharmacokinetics and biodistribution of F@TPSA in B-NHL mouse model
Existing fludarabine injectable formulations have short half-lives, resulting in low bioavailability of FA. At the same time, in view of the wide distribution of lymphoid tissue, the clinical application of FA is mostly systematic administration, but the lack of FA targeting is easy to cause systemic toxicity. Therefore, innovative formulations play a crucial role in improving the aforementioned issues of FA.
The in vitro cell uptake experiments and cytotoxicity experiments have proved that F@TPSA can effectively achieve specific targeting and effective killing of CD22-positive B lymphoma cells, and it is reasonable to expect that F@TPSA can have good application in animal models. Therefore, B-NHL mouse model based on Raji cells was established, and the pharmacokinetic characteristics and biological distribution of supramolecular nanoprodrug F@TPSA in tumor-bearing mice were first investigated. Briefly, Raji cells were inoculated subcutaneously in SCID mice to establish a B-NHL model (Fig. 9A). When the tumor volume reaches 100 mm3 (approximately 14 days), a single intravenous dose of free FA or F@TPSA (both at 100 mg/kg FA) is administered. The concentration of the FA in the blood is monitored at a predetermined time point. After 24 h, major organs of mice were collected for drug concentration detection.
Pharmacokinetics and biodistribution studies. A Protocol for B-NHL (Raji cell) xenograft model and monitor process. B The drug concentration of different FA formulations in the plasma of B-NHL xenograft mice model. C Cumulative amount of FA in plasma by calculating the area under the curve (AUC). D The FA content in major organs of tumor-bearing mice treated with FA or F@TPSA after 24 h. All data are presented the mean ± SD (n = 6). *p < 0.05, ***p < 0.001
Figure 9B shows the curve of drug concentration in the blood of tumor bearing mice over time. It can be seen that the FA concentrations in plasma of both Free FA and F@TPSA group presented a rapid increase, followed by a decreasing trend, which is consistent with the common phenomenon of intravenous injection. We know that nanoparticles are easily captured and eliminated by the reticuloendothelial system in vivo, but it is worth mentioning that the first half-life of F@TPSA (about 5.7 h) is longer than that of Free FA (about 2.5 h). This is also consistent with previous reports that endogenous PSA has immune escape and long circulation characteristics. In addition, although there was no significant difference in the maximum blood drug concentration (Cmax) between Free FA and F@TPSA, the Free FA group mice reached their Cmax at 0.5 h, while the F@TPSA group was delayed to around 1.5 h. This means that F@TPSA can reduce the sudden release of drugs in the body, thereby avoiding some of the side effects. It was precisely due to these differences in pharmacokinetic parameters that the area under the drug-time curve (AUC) of F@TPSA was about 1.44 times that of the Free FA group, reflecting a significant difference in the accumulation of drugs in the circulation system (Fig. 9C).
Furthermore, except pharmacokinetics, the distribution of F@TPSA in major organs (except heart, liver, and lung) was also different from that of Free FA (Fig. 9D). Most excitingly, 24 h after injection of F@TPSA, the amount of the drug in the tumor tissue was 2.1 times higher than in the Free FA group. This is also consistent with the trend of results in vitro cell experiments (Fig. 7), indicating that F@TPSA has the ability to target B-NHL in vivo. On the contrary, FA is thought to be excreted in urine and has certain nephrotoxicity. Therefore, the drug content in the kidneys of mice in the Free FA group was higher than that of F@TPSA, suggesting that F@TPSA would be less nephrotoxic. In addition, it is worth noting that drug concentration in the spleen of the F@TPSA group was about 17% higher than that of the Free FA group. This may be due to the fact that the spleen, as the largest lymphoid organ (55%–60% of lymphocytes are B cells), has a certain specific absorption of F@TPSA, suggesting that we should pay attention to the off-target effects of F@TPSA on the spleen.
Evaluation of antitumor effect and leukopenia of F@TPSA in vivo
These results prove that F@TPSA has a significant features of prolonged circulation time and excellent tumor targeting ability in B-NHL mouse model constructed with Raji cells, which provides the basis for the accurate and efficient treatment of F@TPSA for this disease. Therefore, the same B-NHL mouse model was established and the curative effect of F@TPSA was observed. Briefly, tumor tissue volume was measured and calculated at a predetermined time point after receiving 3 injections of Free FA or F@TPSA (Fig. 10A). On the 40th day, tumor tissues of sacrificed mice were collected and sliced for observation by HE and Ki-67 staining.
The inhibitory effect of F@TPSA on the B-NHL xenograft mice model. A Establishment of B-NHL xenograft mice model and treatment regimen. At a dose of 100 mg/kg FA. B Tumor growth curves after various treatment. C On the 40th day, the tumor-bearing mice were sacrificed, tumor tissue were removed, and their volume was measured and calculated. D, E Representative microscopic images of Ki-67 immunohistochemical staining in tumor tissue sections (D) and proportion of Ki-67 positive cells (E). F Representative optical microscope image of H&E staining of tumor tissue sections. G, Leukocytes in a femur were collected and counted at 40th day. All data are presented the mean ± SD (n = 6). ***p < 0.001
The Fig. 10B shows the changes in tumor volume of tumor-bearing mice within 40 days. Obviously, the tumor volume of the three groups showed a significant difference in growth rate after the 20th day. The growth rate of saline group was the highest, while both Free FA and F@TPSA could inhibit the increase of tumor volume, while F@TPSA had a stronger inhibitory effect. Specifically, from Fig. 10C, by the 40th day, the tumor volume of saline group mice was 3.4 ± 0.2 cm3, while the Free FA group and F@TPSA group were 1.7 ± 0.2 and 1.0 ± 0.2 cm3, respectively. There was a significant difference between F@TPSA and Free FA. We know that Ki-67 is an antigen of proliferating cells, and its expression is positively correlated with the proliferation of tumor cells. The Ki-67 immunohistochemical staining photos of tumor tissue slices in Fig. 10D also show that the staining degree (brown) of the saline group is stronger than that of Free Fa and F@TPSA. After the statistics of Image-Pro Plus® software, the proportion of Ki-67 positive cells in the tumor tissues of mice in the Free FA group and the F@TPSA group was 36.6 ± 2.2% and 28.2 ± 2.1%, which were both lower than 54.6 ± 5.3% in saline group (Fig. 10E). This proved that both Free FA and F@TPSA could inhibit the proliferation of tumor cells, but F@TPSA was more effective. Subsequently, H&E staining of tumor tissue sections also suggested that F@TPSA could cause a wider range of tumor tissue cell necrosis than Free FA (Fig. 10F). On the other hand, as a nucleoside drug, the most common side effect of FA is leukopenia, which is also an important factor limiting its clinical application. By counting total leukocytes in a single femur of tumor bearing mice (Fig. 10G), the leukocytes content in the saline treatment group mice is 15.5 ± 3.2 × 106. However, Free FA significantly decreased leukocytes in tumor-bearing mice (only 39.6% of that in saline group), known as leukopenia. Encouragingly, there was only a 33.7% decrease in the F@TPSA group compared to the saline group. This reveals that F@TPSA can reduce the damage of FA to the hematopoietic system through better tumor targeting effect, and thus has better biocompatibility.











Add Comment