A novel chill model applied to the dormancy release of E. brevicornu seeds
CU confirmation
E. brevicornu is a wide ecological amplitude plant. It occurs in the temperate and subtropical forests, thickets and slopes in China [30]. Plants bloom in May to June, and fruits mature from June to August. As seeds spread in summer, they would undergo high-low temperature changes until germination. Thus, we suggest, except for cold stratification, a variable-temperature process that simulates natural condition may also help to release physiological dormancy. So, we firstly monitor germination percentage in seeds in variable-temperature and low-temperature stratification.
As shown in Fig. 1, the germination percentage of E. brevicornu increased with an increasing number of days under each temperature treatment. At 4 °C, the final germination percentage of E. brevicornu seeds treated with chilling for 11 d was 15.56%, while the final germination percentage of E. brevicornu seeds treated with chilling for 104 d was 86.67%. This indicated that the dormancy-releasing effect of low temperature on E. brevicornu seeds accumulated gradually with increasing cold treatment days. The germination percentage reached a maximum of 86.67% after 104 d of chilling at a low temperature of 4 °C. The optimum temperature range was 2-6 °C. Within this temperature range, the final germination percentage of E. brevicornu seeds treated with chilling for 56 d was 84.44% at 4 °C, while the final germination percentage of seeds at 10 °C was only 33.33%. The slope of the curve for each treatment decreased significantly with increasing temperature, and the number of days required to reach the maximum germination percentage increased. During the same chilling treatment period of 104 d, E. brevicornu seeds took 1, 23, 49, 56, and 64 d to reach the maximum germination percentage under the treatments of 2, 4, 6, 8, and 10 °C, respectively.
Chilling unit can be used to describe the efficiency of each cold temperature on releasing seed dormancy and it was studied as follows. In this study, we took the relative slope value of the chilling-response curves (Fig. 1) at each temperature as the chilling unit value of each temperature (unit is CU). A CU model was constructed to describe the contribution of low temperature to the chilling unit. From Fig. 2A, the CU fitting function for the temperatures of 2, 4, and 6 °C was Y = –\(0.1345X^{2}\) + 1.0503X + 6.4583 (\(\text {R}^{2}\) = 1). The optimum temperature for E. brevicornu seed dormancy release was 3.90 °C, and the maximum CU was 8.5087.
Fitting of CU and CA. (A) Fitting of CU at each temperature. CU was fitted at temperatures of 2, 4, and 6 °C, and the best-fit temperature was 3.90 °C. (B) Logistic fitting of CA under different germination days. a, b, c and d show logistic fits of CA at 30, 40, 50, and 60 d of germination, respectively
We define the chilling accumulation for E. brevicornu seeds within an hour at a certain temperature as being equal to the chilling unit for the temperature. Then, the response curve of between chilling accumulation and the germination percentage was constructed, which we named CA model. Using this model, we can develop an accurate chilling strategy for the dormancy release of E. brevicornu seeds. The germination percentage was determined using the equation:
$$\begin{aligned} Germination\,percentage (\%) = \frac{A1 – A2}{1 + \left( \frac{X}{X0} \right) ^P}- A2 \end{aligned}$$
where A1 and A2 are the minimum and maximum germination percentage achieved at a determined number of germination days, X0 is one-half of the CA required to achieve the maximum germination percentage, X is the chilling accumulation, and P is an equation-specific coefficient.
Figure 2B describes the CA model fitted by a logistic curve at the optimum cold-stratification temperature of 3.90 °C. The model describes the relationship between CA and germination percentage at 30, 40, 50, and 60 d of stratification, with \(\text {R}^{2}\) values of 0.8856, 0.9254, 0.9206, and 0.8907, respectively. From the figure, the X0 values at the four germination stages were 1005.6468 ± 100.5069, 806.6170 ± 57.1405, 763.3549 ± 42.5319, and 735.7646 ± 47.5271 CA. If the maximum germination percentage was reached at 30 d after stratification at 3.90 °C, the required stratification time was 2 \(\times\) 1005.6468 CA/ 1 CU = 2011.2936 h. This resulted in the following CA values: 1613.234, 1526.7098 and 1471.5292 if the maximum germination percentage was reached at 40, 50, and 60 d after stratification at 3.90 °C.
Verification of the chilling models
The obtained CU and CA models were validated using E. brevicornu seeds from different harvesting years as experimental materials. As shown in Fig. 3A, \(\text {R}^{2}\) = 0.8228 between the predicted and actual values of germination percentage, and the error band increased in width at low germination percentage (< 30%) and high germination percentage (> 60%), indicating that the CA model obtained by logistic fitting was less accurate in predicting lower versus higher germination percentage of E. brevicornu seeds. As shown in Fig. 3B, the actual germination percentages of E. brevicornu seeds after 30, 40, 50, and 60 d of stratification at the optimum temperature of 3.90 °C were lower than the predicted values.
Verification of chilling models in E. brevicornu seeds. A Correlation between predicted and actual germination percentage in the validation test of CA of E. brevicornu seeds. The red band is the error band, and the blue-purple band is the predicted bands. B Comparison of predicted and actual germination percentages after 30, 40, 50, and 60 d of germination at a temperature of 3.90 °C. Hollow shapes and solid shapes indicate predicted and actual values, respectively. Different geometric shapes indicate different germination days
Variable-temperature or low-temperature stratification alone did not contribute to the dormancy release of E. brevicornu seeds
As shown in Table 1, the embryo rate of E. brevicornu seeds without stratification treatment (0-0) was 6.77%, but the embryo rate reached 18.21% after 4 months of low-temperature stratification (0-4) and 17.7% after 4 months of variable-temperature stratification (4-0). The embryo rates of seeds after 2 months of variable-temperature stratification and 2 months of low-temperature stratification (2-2) or 1 month of variable-temperature stratification and 3 months of low-temperature stratification (1-3) were 35.01% and 40.39%, respectively. The embryo rate of seeds treated with 1-1 stratification increased from 7.44% to 24.73%, and that of seeds treated with 4-1 stratification increased from 17.70% to 62.21%. The seed embryos developed rapidly after the seeds were transferred from variable-temperature stratification to low-temperature stratification for 1 month. The results indicated that variable-temperature stratification and low-temperature stratification alone did not significantly contribute to seed embryo development and that a combination of variable-temperature and low-temperature stratification was required to release seed dormancy in E. brevicornu. To further investigate the effects of different stratification treatments on seed dormancy, we calculated the germination percentages of seeds under different treatments. Figure S1 shows the germination percentages of all variable-temperature-low-temperature stratification combinations at different temperature gradients. The germination percentage of E. brevicornu seeds in the 4-3 stratification combination was the highest among all temperature gradients, reaching 90.0% at 39 d under the 4 °C treatment, followed by the 3-3 and 2-3 stratification combinations.
Clustering analysis divided the nine treatment combinations into three major clusters according to transcriptome data (Fig. 4A). Cluster I included 1-3, 2-3, 3-3 and 4-2, cluster II included 4-1 and 4-3, cluster III included 4-0, 0-0, and 0-3. Three major groups were formed according to the growth status of the seed embryos with dashed lines (Fig. 4B). Group I included 4-0, 0-0, and 0-3, corresponding to Fig. 4B-a, B-b, and B-c, respectively. Group II included 1-3, 2-3, and 3-3, corresponding to Fig. 4B-d, B-e, and B-f, respectively. Group III included 4-1, 4-2, and 4-3, corresponding to Fig. 4B-g, B-h, and B-i, respectively. The results of transcriptome data clustering were not consistent with those of embryo percentage grouping. This indicated possible post-transcriptional and translational mechanisms of regulation of gene expression might exist here, which need further study.
Effect of stratification on E. brevicornu seeds. (A) Clustering analysis of differentially expressed genes among comparison groups. (B) Developmental statuses of E. brevicornu seed embryos with different stratification treatments. a. Globular embryo period, b. globular embryo microstructure, c. heart embryo period, d. torpedo embryo period, e. early cotyledon microstructure, f. mid-cotyledon embryo, g. late cotyledon embryo, h. late cotyledon embryo microstructure, i. germination; seed embryo stained with Safranin O-Fast Green stain. SEM: small endosperm cells; HEM: large endosperm cells; CO: cotyledon; Ra: radicle. The scale bar is 1 mm. The black dashed line shows the embryo phenotype at different stages according to embryo percentage, and the green dashed line shows the outline of embryo shapes
Different seed structures can be distinguished through safranin O-fast green staining. Nonlignified cells appear green from fast green staining, and lignified cells appear magenta from safranin O staining. As seen from the figure, the proembryonal cells of E. brevicornu seeds in the 0-0, 0-3, and 4-0 treatments developed into globular embryos, still at the early stage of seed embryo development; the endosperm occupied the vast majority and clearly developed into two parts, with the part near the embryo being the small embryo cells (SEM) and most of the part away from the embryo differentiating into the large embryo cells (HEM) (Fig. 4Bb). In the microstructure of 1-3, 2-3, and 3-3 cotyledon embryos at the early stage (Fig. 4Be), the SEM inclusions gradually disappeared and formed a clear cavity, while the HEM inclusions remained clearly visible and the cotyledon (CO) started to develop. In the 4-2 and 4-3 treatments, most of the seed embryos developed to the late stage of cotyledon embryo or germination, and the microstructure showed that the SEM completely disappeared and degenerated into a layer of cells surrounding the embryo, the HEM cell layer thinned at the embryo root, and the cotyledons elongated further (Fig. 4Bh).
The first principal component (PC1) explained 16.617% of the characteristics of the original dataset, from which it was found that the 4-0 and 0-3 treatments (which involved only low- or variable-temperature stratification), as well as the control treatment (0-0), were significantly separated from the other treatments. The second principal component (PC2) explained 9.684% of the characteristics of the original dataset. According to the second principal component, the separation between the control treatment (0-0) and the other treatments was significant (Figure S2).
Transcriptome sequencing results reveal the dormancy release mechanism of E. brevicornu seeds
Differentially expressed gene (DEG) filtering
To investigate the dormancy release mechanism of E. brevicornu seeds under different stratification treatments, we selected nine treatments (0-0, 0-3, 1-3, 2-3, 3-3, 4-3, 4-2, 4-1, 4-0) for transcriptome sequencing, with three replicates per treatment, and seeds without stratification treatments were used as controls (0-0). In total, we obtained 191.54 Gb of clean data, and the data for each sample reached 6 Gb. The Q30 base percentage was at least 88%. These statistics suggested that the quality and amounts of the generated reads were sufficient for transcriptome analysis (Table S1). The clean data for 27 samples were assembled using Trinity, resulting in 513793 transcripts and 433387 unigenes. The N50 and mean length of the assembled transcripts were 794 bp and 575 bp, respectively. For the unigenes, the N50 and mean length were 884 bp and 637 bp, respectively (Table S2). A total of 71,990 DEGs (|log2(fold change)| \(\ge\) 1 and FDR < 0.05) were obtained by two-by-two comparisons of seeds from the nine stratification treatments of E. brevicornu (Figure S3).
To investigate the molecular mechanism of variable-temperature stratification on release from physiological dormancy in Epimedium, we selected five comparison groups, 0-0 vs. 4-0, 0-3 vs. 1-3, 0-3 vs. 2-3, 0-3 vs. 3-3, and 0-3 vs. 4-3, for which the total number of DEGs was 2525. To investigate the molecular mechanism of low-temperature stratification on release from physiological dormancy in Epimedium, we selected four comparison groups, 4-0 vs. 4-1, 4-0 vs. 4-2, 4-0 vs. 4-3, and 0-0 vs. 0-3, for which the total number of DEGs was 3340 (Fig. 5A).
Analysis of DEGs between variable-temperature stratification and low-temperature stratification groups. (A) Venn diagram analysis of DEGs. a. Venn diagram analysis of 5 variable-temperature stratification groups; b. Venn diagram analysis of 4 low-temperature stratification groups. (B) DEG KEGG enrichment analysis graph. a. KEGG enrichment analysis of 5 variable-temperature stratification groups; b. KEGG enrichment analysis of 4 low-temperature stratification groups. (C) K-means clustering analysis of DEGs. a. K-means clustering analysis of 5 variable-temperature stratification groups; b. K-means clustering analysis of 4 low-temperature stratification groups. The annotated genes were aligned against Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/), and obtained the appropriate copyright permission to modify the KEGG image depicted in Fig. 5
To identify the biological pathways with which these DEGs may be associated, we annotated the 2525 and 3340 DEGs with the KEGG database (Fig. 5B). The DEGs in both comparison groups of variable temperature and low-temperature stratification were significantly enriched in plant hormone signal transduction (ko04075), starch and sucrose metabolism (ko00500), metabolic pathways (ko01100) and secondary metabolite biosynthesis (ko01110), and the results of the enrichment analysis imply the importance of plant hormone signal transduction and starch-sucrose metabolism pathways for the dormancy release of E. brevicornu seeds.
By K-means clustering analysis, we obtained 5 and 6 clusters across samples in the two stratified comparison groups (Fig. 5C). Among them, clusters 2 and 5 showed the expected DEG accumulation pattern associated with the dormancy release of E. brevicornu seeds: the expression of DEGs gradually increased as the degree of dormancy diminished. Cluster 2 contained 1151 genes, and cluster 5 contained 1764 genes.
Analysis of plant hormone signal transduction and starch-sucrose metabolism pathways
Interestingly, gene expression in cluster 2 and cluster 5 increased with dormancy release, and the results of the enrichment analysis implied the importance of plant hormone signal transduction and starch-sucrose metabolism pathways for dormancy release in E. brevicornu seeds. Therefore, we performed a detailed analysis of phytohormone and starch-sucrose metabolism-related genes in cluster 2 and cluster 5.
The accumulation patterns of 1330 and 1585 DEGs detected in cluster 2 and cluster 5, respectively, were consistent with the degree of dormancy release in E. brevicornu seeds. Among them, 42 and 33 DEGs belonged to the plant hormone signal transduction pathway (Fig. 6A), and 40 and 32 DEGs belonged to the starch and sucrose metabolism pathway (Fig. 6B). A total of 42 significant DEGs were identified in plant hormone signal transduction pathways in the variable-temperature stratification group (Fig. 6A). Four enzyme-encoding genes, including the auxin transporter gene AUX1, the auxin receptor gene TIR1, the early auxin response gene AUX/IAA, and ARF, were identified in the IAA pathway. Three enzyme-encoding genes, including the histidine-containing phosphotransfer protein gene (AHP), a two-component response regulator ARR-B-family gene (B-ARR), and a two-component response regulator ARR-A-family gene (A-ARR), were identified in the cytokinin (CTK) pathway. DELLA was identified in the GA pathway. Two enzyme-encoding genes were identified in the ABA pathway, including the abscisic acid receptor PYR/PYL-family gene PYR/PYL and the PP2C gene. The gene encoding the enzyme mitogen-activated protein kinase 6 (MPK6) was identified in the ethylene (Eth) pathway. Four enzyme-encoding genes, including BR-insensitive 1 (BRI1), BRI1 kinase inhibitor 1 (BKI1), TCH4, and cyclin D3 (CYCD3), were identified in the BR pathway. Two enzyme-encoding genes, including JA ZIM domain-containing protein (JAZ) and the TF MYC2, were identified in the JA pathway. Two enzyme-encoding genes, including the regulatory protein NPR1 and pathogenesis-related protein 1 (PR-1), were identified in the salicylic acid (SA) pathway.
Expression of each hormone-related transcript in E. brevicornu seeds of different treatment groups. (A) Expression of each hormone-related transcript in E. brevicornu seeds of the variable-temperature stratification group. (B) Expression of each hormone-related transcript in E. brevicornu seeds of the low-temperature stratification group. a. IAA; b. CTK; c. GA; d. ABA; e. ethylene; f. BR; g. JA; h. SA
A total of 33 significant DEGs were enriched in plant hormone signalling pathways in the low-temperature stratification group (Fig. 6B). Five enzyme-encoding genes whose expression differed in the low-temperature treatment compared to the variable-temperature treatment included ARF in the IAA pathway, the CTK receptor CRE1 in the CTK pathway, the serine/threonine-protein kinase CTR1 in the Eth pathway, and BR-signalling kinase (BSK) and brassinazole-resistant 1/2 (BZR1/2) in the BR pathway.
Sucrose is a synthetic precursor of starch in developing seeds [31]. The expression of genes related to starch and sucrose metabolic pathways is shown in Fig. 7. The morphological dormancy of E. brevicornu seeds was gradually released as the stratification time increased. Sucrose synthase (SUS, EC:2.4.1.13) in seeds is usually associated with starch accumulation and can indirectly promote relatively highly efficient starch biosynthesis by increasing the levels of the source of UDP-glucose (UDPG) [32, 33]. Meanwhile, glucose 6-phosphate is regulated by two enzymes, phosphoglucomutase (pgm, EC:5.4.2.2) and glucose-1-phosphate adenylyltransferase (glgC, EC:2.7.7.27), for the progressive synthesis of ADP-glucose (ADPG) and amylose. Increasing the concentrations of hexoses such as UDPG and ADPG induces the lengthening of long straight-chain starch chains by granule-bound starch synthase (WAXY, EC:2.4.1.242) and eventually by 1,4-\(\alpha\)-glucan branching enzyme (glgB, EC:2.4.1.18), which synthesize amylose into starch, and the transcript levels of both enzymes were highest in the 4-3 treatment. During starch metabolism, the enzyme glycogen phosphorylase (glgP, EC:2.4.1.1) exerts feedback regulation, causing glycogen to be catabolized to \(\alpha\)-glucose-1P upon accumulation of starch content.
Much glucose needs to be stored prior to starch synthesis to stimulate cell reproduction and delay differentiation [34], and as seed dormancy is released, glucose synthesis activity increases. UDPG generates 1,3-\(\beta\)-glucan as a major component of the cell wall via 1,3-\(\beta\)-glucan synthase, which in turn synthesizes glucose from glucan endo-1,3-\(\beta\)-D-glucosidase (EGLC, EC:3.2.1.39). The transcript expression of \(\beta\)-glucosidase gradually increased with the stratification time of E. brevicornu seeds and was highest under 4-3 stratification. The enzyme \(\beta\)-fructofuranosidase (SacA, EC:3.2.1.26) catabolizes sucrose directly into glucose, and its transcript level peaked under 2-3 stratification along with the dormancy release of E. brevicornu seeds. Starch was also decomposed with stratification time due to activation of isoamylase (ISA, EC:3.2.1.68) and \(\alpha\)-amylase (AMY, EC:3.2.1.1) to produce soluble oligosaccharides, which were then instantly catabolized by \(\beta\)-amylase into maltose and eventually by the enzyme 4-\(\alpha\)-glucanotransferase (malQ, EC:2.4.1.25) gene into glucose. As shown in the figure, \(\beta\)-amylase transcript levels remained low in the 4-0 treatment, verifying the speculation that a single variable-temperature stratification did not release the dormancy of E. brevicornu seeds.
Analysis of metabolic pathways of embryo development ending in seed dormancy and fatty acid degradation during dormancy
To explore more deeply and systematically the DEGs associated with the dormancy release of E. brevicornu seeds, we clustered 71990 DEGs, 57011 of which were excluded due to unclear expression patterns. The resulting total of 14979 genes are displayed in Fig. 5. These genes were divided into 17 clusters (Fig. 8A). Among them, clusters 1, 2, 3, 7, 11, 12, 13, 14, and 16 contained genes that were individually highly expressed in different stratification treatments, and cluster 5 contained genes that were coexpressed in the combined variable-temperature-low-temperature stratification. Fifteen of the 17 clusters were significantly enriched with genes, while cluster 12 and cluster 17 did not have significantly enriched genes (Fig. 8B). Cluster 2 contained the most genes, which were individually highly expressed in the 4-0 treatment, while cluster 1 contained the fewest genes, which were individually highly expressed in the 4-2 treatment. Cluster 2 contained the most TFs, and cluster 1 contained the fewest TFs.
The genes in cluster 5 were co-highly expressed in the combined variable-temperature-low-temperature stratification. To illustrate the effect of combined stratification in relieving E. brevicornu seed dormancy, we analysed the biological pathways associated with cluster 5 and found that two pathways, embryo development ending in seed dormancy and fatty acid degradation, were significantly enriched.
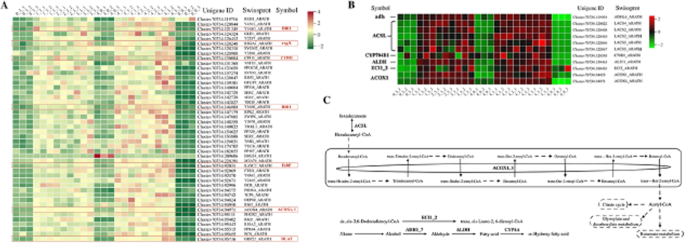
As shown in Fig. 9A, a total of 35 genes associated with embryo development ending in seed dormancy (GO:000573) were selected. The transcript levels of these genes were significantly higher in the combination stratification than in the treatment groups that were treated only with low or variable temperature. Among them was BRI1, which senses and combines with BR; sterol 14\(\alpha\)-demethylase (CYP51, EC:1.14.14.154), which is involved in the biosynthesis of phytosterols and BRs [35]; thiamine phosphate phosphatase (rsgA, EC:3.1.3.100), which is involved in the regulation of endogenous GA content and is commonly expressed in actively growing and elongating plant tissues [36]; 3-oxoacyl- synthase II (fabF, EC:2.3.1.179), which is associated with \(\alpha\)-linolenic acid metabolism; pyruvate dehydrogenase E2 component (DLAT, EC:2.3.1.12), which is associated with the first step of fatty acid biosynthesis; and acyl-CoA oxidase (ACOX1, 3, EC:1.3.3.6), which is the main enzyme of the fatty acid \(\beta\)-oxidation pathway.
A total of six genes related to fatty acid degradation (ko00071) were filtered, as shown in Fig. 9B. Fatty acid degradation begins with long-chain acyl-CoA synthetase (ACSL, EC:6.2.1.3), which activates the transformation of free fatty acids into acyl-CoA; this step is followed by a \(\beta\)-oxidation cycle that serially breaks down acetate units [37]. The expression of alcohol dehydrogenase (adh, EC:1.1.1.1), which is involved in fatty acid \(\beta\)-oxidation, and ACOX1 was significantly higher in the combined stratification treatment groups (1-3, 2-3, 3-3, 4-1, 4-2, 4-3) than in the treatment groups that were stratified only by variable temperature or low temperature (0-3, 4-0) versus the control group (0-0) (Fig. 9B). Oxidation of hexadecanoic acid to acetyl-CoA, followed by butanoate metabolism, glyoxylate and dicarboxylate metabolism, and citrate metabolism pathways, provided energy for the dormancy release of E. brevicornu seeds (Fig. 9C).
Gene coexpression analysis
In this study, coexpression analysis of 4709 DEGs in cluster 5 with 230 TFs was performed to infer the reciprocal regulatory relationships between genes. To ensure strong interconnections among genes, the top 100 pairs of reciprocal relationships with absolute values of correlation coefficients \(\ge\) 0.96 were screened for in-depth analysis.
Figure10 shows the core network of coexpression analysis, which included 11 TFs and 69 genes. In this network, GRF TFs had the most interactions, interacting with 12 genes. Next were BAF and MYB TFs, which interacted with 9 genes. The others were AUX/IAA and ARF, YABBY, C3H and KNOX, HD-Zip and zf-HD, and TCP, which had interactions with 8, 6, 5, 4, and 3 genes, respectively. The TFs zf-HD and YABBY also interacted with each other, as did MYB, GRF and TCP, with correlation coefficients greater than 0.98 (Figure S4).














Add Comment