Search results
From the search, we obtained 2858 citations, with 1221 identified as duplicates. Upon screening titles and abstracts, we excluded 1609 articles, retaining 28 for a full-text review. Out of these, 12 RCTs met our eligibility criteria and were included in this systematic review [14,15,16, 18,19,20,21, 23,24,25,26,27]. Of these, eight were subjected to quantitative analysis [15, 16, 18, 19, 21, 25,26,27]. Qualitative analysis was performed for four RCTs. Three RCTs did not report the incidence of OHSS and serum E2 levels which could be pooled into meta-analysis and the authors have not replied to our emails [14, 23, 24]. Another RCT compared the efficiency of letrozole for the prevention of OHSS with ganirelix acetate [20]. A flow chart illustrating the search and selection process is displayed in Fig. 1.
Characteristics of included studies
The twelve RCTs comprised 569 participants in the treatment group and another 524 in the control group. Letrozole was administered in the treatment group during COS in seven RCTs [14, 15, 18, 19, 21, 25, 27], whereas in the early luteal phase in five RCTs [16, 20, 23, 24, 26]. The participants were treated with gonadotrophin-releasing hormone (GnRH) antagonist protocol in five RCTs [14, 18, 19, 21, 25], GnRH agonist long protocol in six RCTs [15, 16, 20, 23, 24, 27], and GnRH agonist protocol in one RCT [26]. The freeze-all and fresh transfer strategy was used in seven RCTs [16, 19,20,21, 23, 24, 26] and five RCTs [14, 15, 18, 25, 27], respectively. Eight RCTs reported the incidence of OHSS [16, 18,19,20,21, 25,26,27]. Five RCTs utilized diagnostic criteria for OHSS [4, 8, 28, 29] that were internationally recognized, albeit with slightly variations [16, 19,20,21, 26]. However, three RCTs did not describe the criteria for OHSS diagnosis [18, 25, 27]. Six RCTs investigated the efficacy of letrozole in preventing moderate to severe OHSS and specifically severe OHSS [16, 19,20,21, 25, 26]. Among them, four RCTs compared the incidence of OHSS in patients treated with letrozole versus placebo or no treatment [16, 19, 21, 25]; one RCT compared letrozole with aspirin, which was considered as a placebo [26]; and the other one RCT evaluated the effect of letrozole versus ganirelix acetate (gonadotropin-releasing hormone antagonist) in OHSS prevention [20]. Five RCTs reported the incidence of mild OHSS after letrozole treatment [19,20,21, 25, 26]. Serum E2 levels were evaluated in all RCTs. Seven RCTs used letrozole during COS, and all of them reported serum E2 levels on hCG trigger day [14, 15, 18, 19, 21, 25, 27]. Five RCTs used letrozole in early luteal phase [16, 20, 23, 24, 26], among them, two RCTs reported serum E2 levels on the 4th, 7th and 10th day after hCG trigger [23, 24]; one RCT reported serum E2 levels on the 7th day after hCG trigger [26]; one RCT displayed the trend of serum E2 levels on the 5th, 8th and 10th day after hCG trigger without reporting specific E2 values [16]; and one RCT reported serum E2 levels on the 5th and 7th day after oocyte retrieval [20]. The characteristics of the included studies are summarized in Table 1.
Risk of bias in included studies
We assessed the risk of bias for all included studies. In terms of performance bias and detection bias, we assessed the risk based on the reported outcome of OHSS incidence; if not reported, we evaluated the outcome of E2 levels. Eight RCTs provided clear methods for random sequence generation. They used methods like computer-generated randomization list, block randomization, or drawing lots, leading them to be rated as having a low risk of bias [15, 19,20,21, 23, 24, 26, 27]. The remaining four RCTs were judged as unclear risk due to lack of relative information [14, 16, 18, 25]. Three RCTs were at low risk of bias for allocation concealment as the sealed envelope method was used to randomly allocate patients into two groups [14, 25, 27]. Nine RCTs providing no detailed information were judged as unclear risk of allocation concealment [15, 16, 18,19,20,21, 23, 24, 26]. We consider that whether using blinding of participants and personnel was less likely to exert effects on any outcomes evaluated by this review, so all RCTs were assessed as low risk of bias in this domain [14,15,16, 18,19,20,21, 23,24,25,26,27]. Four RCTs had a low risk of detection bias, as they exclusively reported outcomes related to of E2 levels, determined through automated analysis [14, 15, 23, 24]. The rest eight RCTs reported the outcome of OHSS incidence involving subjective judgement, but these trials did not mention blinding of outcome assessors, hence they were at unclear risk of bias [16, 18,19,20,21, 25,26,27]. Nine RCTs did not reported any losses to follow-up [16, 18,19,20,21, 23,24,25, 27], and two RCTs, providing missing data and reasons for discontinuation, were balanced between groups [14, 15], therefore they were rated as low risk of attrition bias. The other one RCT was judged as high risk in this domain because it excluded more than 10% patients from the analysis and there was a clear difference in the proportion of missing between the treatment and control groups [26]. Six RCTs were at low risk of reporting bias due to the fact that they reported the incidence moderate to severe OHSS [16, 19,20,21, 25, 26]. Six RCTs did not reported this primary outcome, so they were considered as unclear risk [14, 15, 18, 23, 24, 27]. The other biases were assessed at last. In seven out of the eight RCTs which reported the incidence of OHSS, there were no significant differences in major baseline characteristics, including female age, body mass index (BMI), ovarian reserve markers (anti-Müllerian hormone, antral follicle count, baseline follicle stimulating hormone or the combination of two or three aforementioned markers), and number of oocytes retrieved between the letrozole and control groups [16, 19,20,21, 25,26,27]. Only one RCT was rated as unclear risk of this domain because the authors did not report BMI, number of oocytes retrieved or number of mature follicles and have not replied to our email [18]. In addition, three RCTs did not describe the diagnostic criteria of OHSS [18, 25, 27]; five RCTs recruited patients with high risk of OHSS, so there was a possible contamination bias [16, 19,20,21, 26]. Therefore, these RCTs are at unclear risk of other biases [16, 18,19,20,21, 25,26,27]. The assessment of the “risk of bias” based on Cochrane’s criteria is shown in Fig. 2A and B.
Primary outcome
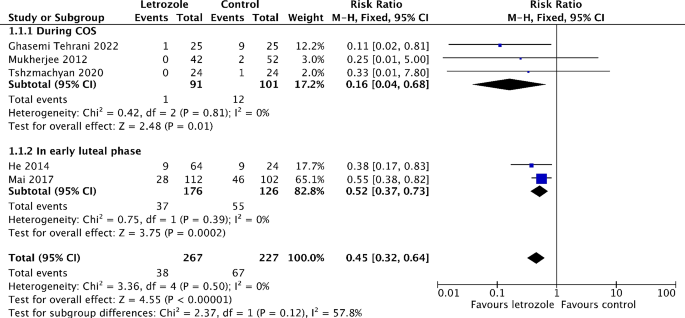
Incidence of moderate to severe OHSS
Six studies reported on the incidence of moderate to severe OHSS [16, 19,20,21, 25, 26]. Out of these, five studies made comparison between letrozole and either a placebo or no treatment [16, 19, 21, 25, 26]. The meta-analysis showed that the risk of moderate to severe OHSS significantly reduced by 55% in the letrozole group (RR 0.45, 95% CI 0.32 to 0.64, I2 = 0%, 5 RCTs, 494 patients) (Fig. 3). The subgroup analysis revealed the risk of moderate to severe OHSS is lower when letrozole was administered during COS (RR 0.16, 95% CI 0.04 to 0.68, I2 = 0%, 3 RCTs, 192 patients), compared to in the early luteal phase (RR 0.52, 95% CI 0.37 to 0.73, I2 = 0%, 2 RCTs, 302 patients) (Fig. 3). The similar results were obtained when only RCTs recruiting participants with high risk of OHSS are pooled in meta-analysis (Figure S2). In addition, the risk of moderate OHSS significantly decreased by 55% in the letrozole group (RR 0.45, 95% CI 0.31 to 0.66, I2 = 0%, 5 RCTs, 494 patients) (Figure S3). Although not significant, there was a trend toward a lower risk of severe OHSS in the letrozole group (RR 0.47, 95% CI 0.19 to 1.18, I2 = 0%, 2 RCTs, 494 patients) (Fig. 4). One study has not been included in the meta-analysis as it compared the efficiency of letrozole for the prevention of OHSS with ganirelix acetate [20]. Letrozole might be more effective than ganirelix acetate in preventing moderate OHSS, although the difference of incidence was not statistically significant (9.8% vs. 16.3%, P = 0.38). Both drugs displayed equivalent efficacy in severe OHSS prevention (3.3 vs. 3.3%, P = 1.00).
Secondary outcomes
Incidence of overall OHSS
Eight studies reported on the incidence of overall OHSS [16, 18,19,20,21, 25,26,27]. Of these, seven studies made a comparison between letrozole and either a placebo or no treatment [16, 18, 19, 21, 25,26,27]. The meta-analysis showed that the risk of overall OHSS significantly reduced by 53% in the letrozole group (RR 0.47, 95% CI 0.23 to 0.97, I2 = 74%, 7 RCTs, 724 patients) (Fig. 5). Notably, the subgroup analysis revealed that only when letrozole was administered during COS, the risk of overall OHSS significantly reduced(RR 0.43, 95% CI 0.24 to 0.79, I2 = 0%, 5 RCTs, 422 patients) (Fig. 5). Another study reported a reduced incidence of overall OHSS in the letrozole group when compared to that in the ganirelix acetate group (13.1% vs. 19.6%, P = 0.33) [20].
Incidence of mild OHSS
Five studies reported on the incidence of mild OHSS [19,20,21, 25, 26], and four of them compared letrozole with either placebo or no treatment [19, 21, 25, 26]. The meta-analysis showed that there is a trend toward lower risk of mild OHSS with the administration of letrozole, although not significant (RR 0.71, 95% CI 0.27 to 1.89, I2 = 66%, 4 RCTs, 406 patients) (Fig. 6). Another study was excluded from meta-analysis as the treatment and control groups received letrozole and ganirelix acetate, respectively [20]. And none of mild OHSS cases were reported in this study.
Incidence of critical OHSS
Two studies reported on the incidence of critical OHSS [19, 26]. There were no cases of critical OHSS in both the treatment and control groups of these two studies.
Serum E2 levels
All twelve studies reported on serum E2 levels [14,15,16, 18,19,20,21, 23,24,25,26,27]. Seven studies reported serum E2 levels on hCG trigger day with the administration of letrozole during COS [14, 15, 18, 19, 21, 25, 27]. However, two of them were excluded from the meta-analysis because they did not present the outcome in the format of mean ± standard deviation [14, 27]. The meta-analysis showed that serum E2 levels on hCG trigger day were significantly lower in the treatment group (MD -847.23, 95% CI -1398.00 to -296.47, I2 = 93%, 5 RCTs, 374 patients) (Fig. 7). Five studies used letrozole in the early luteal phase [16, 20, 23, 24, 26]. Among them, two studies showed that serum E2 levels on the 4th (272 ± 65.4 vs. 749 ± 27.4 pg/ml and 279 vs. 1586 pg/ml), 7th (229 ± 69 vs. 1457 ± 152 pg/ml and 240 vs. 855 pg/ml) and 10th (31 ± 7 vs. 1308 ± 88 pg/ml and 40 vs. 448 pg/ml) day after hCG trigger significantly decreased in the letrozole group [23, 24]. However, the meta-analysis was not conducted as one of these two studies did not present the outcome as mean ± standard deviation [24]. Moreover, another study also reported that serum E2 levels on the 7th day after hCG trigger were lower in the letrozole group than those in the control group [84.0 (15.0-223.5) vs. 3110.5 (113.3-4976.8) pg/ml] [26]. One study reported that there was a significant trend toward lower E2 levels in the letrozole group on the 5th, 8th and 10th day after hCG trigger without reporting specific E2 values (P < 0.05) [16]. In addition, similar to the above results, one study found that serum E2 levels decreased on the 5th (685.74 ± 1066.55 vs. 2364.82 ± 1774.25 pg/ml) and 7th (218.38 ± 308.41 vs. 819.67 ± 848.14 pg/ml) day after oocyte retrieval when letrozole was compared with ganirelix acetate [20].









Add Comment