Patients
Patient demographics are shown in Table 1. Within all cohorts, pre-therapeutic mean bilirubin and CA 19 − 9 levels were significantly raised in PDAC samples compared to the benign samples (p < 0.05). Tumour staging was predominantly T4 in both the discovery and technical validation cohort (100% and 67%, respectively), while in the clinical validation cohort staging was mostly T3 (46% of PDAC and 60% of CCA tumours). Most cancers causing biliary obstruction were anatomically located in the head of pancreas (81%).
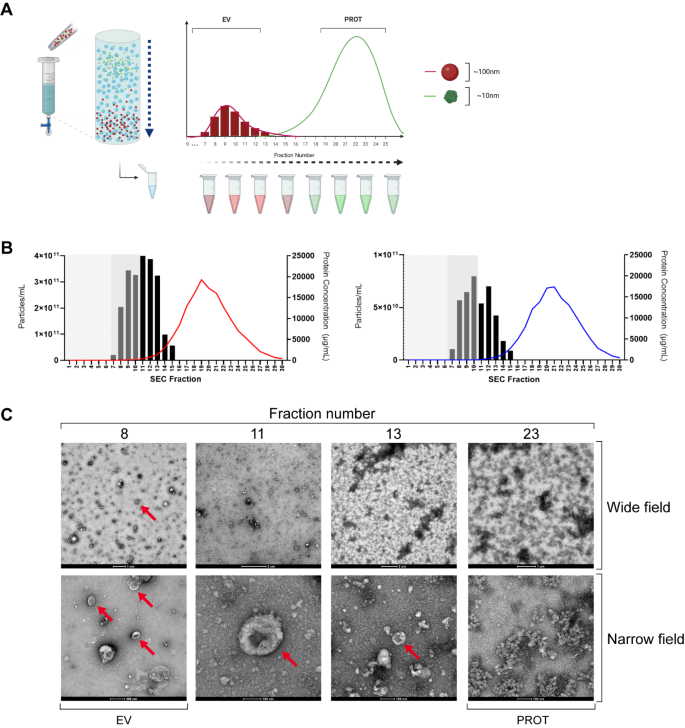
Nanoparticle tracking analysis, electron microscopy and western blotting demonstrate that sec is able to isolate plasma extracellular vesicles
SEC makes use of a stationary phase (a gel) which allows a liquid mobile phase (sterile filtered PBS) to pass through. Plasma samples containing complex molecules such as proteins, lipoproteins and EVs are separated and leave the column at a rate proportional to their hydrodynamic volume. With complex mixtures made up of different sized particles, larger molecules are excluded from the gel and are recovered quickly, whilst smaller particles are impeded and elute much later (Fig. 2A).
During characterisation, 1 mL of each plasma sample (3 PDAC, 3 benign) was loaded to the column and eluted with PBS, which was collected in sequential 500 µL fractions. NTA and bicinchoninic acid assays (BCA) were performed, giving average particle and protein concentrations for each fraction, as shown in Fig. 2B. This figure demonstrates a peak in measured particle concentration at fraction 10–11, which corresponds with the expected EV fractions, in both PDAC and benign samples. Furthermore, maximum measured protein concentrations were 19 ± 2.8 mg/mL in PDAC (Fig. 2B, left), and 17 ± 0.3 mg/mL in benign disease (Fig. 2B, right). Transmission electron microscopy (TEM) images of several fractions of a PDAC sample are shown in Fig. 2C. Red arrows highlight a significant number of EVs (particularly in fraction 8) with the expected cup-shaped morphology that occurs when EV preparations are fixed and dried. As the fraction number increases, so does the presence of non-vesicular protein until EVs are poorly resolved at Fraction 23. Additionally, TEM images of a benign plasma sample are included in Supplementary Fig. 1.
SEC-isolation of plasma EVs. (A) SEC using Izon column separates EVs with a size discrimination of 70–100 nm and was performed for a characterisation of 3 PDAC and 3 benign samples. A volume of 1 mL of plasma was loaded to the column and PBS continuously added with 500 µL fractions eluted for subsequent analysis. The column void volume was approximately 3 ml and protein concentrations were measured using a BCA assay. (B) Particle and protein concentrations were obtained by NTA and BCA for each of the fractions shown and averages for each fraction are shown in (left) PDAC and (right) benign disease. Mean particle concentrations are shown as columns with protein levels shown as a coloured trendline (red – PDAC, blue – Benign). (C) TEM of a representative PDAC sample shows EVs (red arrows). Images taken at high magnification (60,000–72,000x) are labelled as ‘Narrow field’ whilst intermediate magnification (4-5000x) are labelled as ‘Wide field’. BCA: bicinchoninic acid; EV: extracellular vesicle; NTA: nanoparticle tracking analysis; PBS: phosphate buffered saline; PDAC: pancreatic ductal adenocarcinoma; SEC: size exclusion chromatography; TEM: transmission electron microscopy
Western blot analyses were performed for 6 samples (3 benign and 3 malignant) using antibodies against EV markers ALIX and TSG101, as well as lipoprotein marker APOA-1 (Fig. 3) in accordance with the MISEV2018, and MISEV2023 update [28, 29]. ALIX and TSG101 are cytosolic proteins which are associated with endosomal sorting complexes required for transport (ESCRT) machinery. ESCRT machinery is involved in the transport of endosomes to the membrane for EV release. In the benign samples (Fig. 3A), ALIX and TSG101 were present in pooled fractions 7–10, and absent from fraction 11 onwards. Conversely, lipoprotein marker APOA-1 appeared throughout the fractions. Although the hypothesis was a complete absence of these protein markers in fractions 7–10, this was found not to be the case. Therefore, although they can be considered as vesicular preparations, they may contain small amounts of additional plasma constituents. In the malignant samples (Fig. 3B), EVs from the malignant samples showed differential (and heterogenous) protein expression consistent with previous studies by both mass spectroscopy [30], and flow cytometry [31]. Results are consistent regarding ALIX expression in PDAC EVs, as well as a relative absence of APOA-1. TSG101 was not detected in these PDAC EVs. Altogether, these experiments show that SEC is able to isolate plasma EVs and provide sufficient data for the assessment of small RNA biomarkers.
Western blots of specific markers for evaluation of plasma-derived EV preparations. Well-known EV-specific markers, TSG-101 and ALIX, as well as lipoprotein marker APOA-1, were used to assess the purity of the EV preparations. Displayed are (A) 3 benign samples (B1, B2, B3), and (B) 3 malignant samples (M1, M2, M3). Total protein was stained for normalisation. Variable expression is shown, which is consistent with previously published findings from clinically-obtained samples. EV: extracellular vesicle; PROT: protein
EV characterisation showed that EVs are larger and more numerous in PDAC samples
A total of 61 samples (n = 23 benign, 8 CP and 30 PDAC) were analysed by NTA to evaluate EV-characteristics, including median particles size and particle concentration. Supplementary Fig. 2A, shows an example of particle size distribution in a representative (left) PDAC and (right) benign sample. Average particle size (measured as median) was 156.5 nm (± 13.8) across all samples. While PDAC samples showed a mean particle size of 163.0 nm (± 11.2), benign samples showed a smaller mean particle size of 150.3 nm (± 13.4; Supplementary Fig. 2B, left). The EV concentrations followed a skewed distribution, thus median values were calculated. The median EV concentration across all samples was 4.2 × 1010 particles/mL (range: 3.5 × 109 – 1.6 × 1012), which gives an estimated average yield of 8.4 × 1010 EVs/mL of plasma. For PDAC samples, the median EV concentration was 7.7 × 1010 particles/mL (range: 3.5 × 109 – 5.2 × 1011) whilst in benign disease, the median EV concentration was lower at 2.4 × 1010 particles/mL (range: 4.9 × 109 – 1.6 × 1012; Supplementary Fig. 2B, right). When comparing PDAC and benign samples, there was a significant difference in EV concentrations (p = 0.0013) and particle size (p = 0.0002). Although this was a statistically significant difference, the biological significance of this difference is unclear due to the spread and overlap, as discussed previously in the literature [32]. To determine the presence of RNAs suitable for sequencing after extraction from pooled EV fractions, RNA was quantified by automated electrophoresis using an Agilent Bioanalyzer. Plots show a predominance of small RNAs < 200 nucleotides with concentrations ~ 100 pg/µL (Supplementary Fig. 2C).
Small RNA sequencing of plasma-derived EVs reveals differentially expressed MiRNAs and a PDAC associated signature
To discover differentially expressed miRNAs, small RNA sequencing was performed for 10 benign and 10 PDAC samples. An average of 13,004,616 total reads (range: 8,402,014–22,524,635) were found and available for differential expression analysis. Reads were mapped and analysis revealed a high variability in mappable small RNAs (Fig. 4A): 37.2% was made up by miRNAs, followed by 27.2% messenger RNAs (mRNA), 18.6% genomic RNAs, 9.6% ribosomal RNAs (rRNA), and 3.4% transfer RNAs (tRNA). Principal component analysis was used to demonstrate variability between data points (Fig. 4B). Two PDAC samples showed low miRNA composition and were hence excluded from further analysis. To reduce noise, miRNAs were filtered by expression (i.e. minimally expressed in 50% of the samples). The top 10 miRNAs with highest expression in plasma EVs across all samples included members of the let-7 family and oncogenic miRNAs, such as miR-21 (see Table 2) [33].
Small RNA sequencing of plasma-derived EVs. (A) Relative distribution according to small RNA subtype of mapped reads for each sample shown as stacked barplots. (B) Principal component analysis of samples shows clustering of PDAC and benign samples. (C) Volcano plot with 15 differentially expressed miRNAs. Red indicates FDR < 0.05. Vertical lines indicate log2 fold change = ± 1. The 10 most significant miRNAs are labelled as shown. (D) A heatmap shows significant miRNAs (12 upregulated and 3 downregulated) in the pairwise comparison PDAC vs. benign disease, with relative expression calculated and shown. Red indicates upregulated in the sample, while blue indicates downregulated in the sample. EV: extracellular vesicle; mRNA: messenger RNA; miRNA: microRNA; PDAC: pancreatic ductal adenocarcinoma; FDR: false discovery rate; rRNA: ribosomal RNA; tRNA: transfer RNA
Global profiling for miRNAs was undertaken with cut-offs FDR < 0.05 and log2 fold change > 1. Overall, 12 upregulated and 3 downregulated miRNAs were found to be statistically differentially expressed between PDAC vs. benign disease (Fig. 4C). A heatmap of these miRNAs shows clustering of the PDAC samples (Fig. 4D). The 12 upregulated miRNAs in PDAC (Table 3) included the miR-200 family, consisting of miR-141-3p, miR-200a-3p, miR-200b-3p, miR-200c-3p and miR-429. Upregulated miRNAs are deemed detectable in cancer samples and may be more suitable for diagnostics. MiRNAs were selected for further evaluation based on log2foldchange > 5. Therefore, the 5 upregulated miRNAs of the miR-200 family were taken forward as potential candidates to the validation stage for RT-qPCR.
Small RNA sequencing of plasma-derived circulating miRNAs fails to show significant differential expression
As part of a discovery study into diagnostic miRNAs to discriminate PDAC from benign patients, both plasma EV-derived miRNAs and plasma cell-free miRNAs (cf-miRNA) were assessed (Supplementary Fig. 3). Direct small RNA sequencing of miRNAs from the total plasma was performed in 41 samples for the pairwise comparison of malignant vs. benign disease. After controlling for multiple comparisons, two cf-miRNA candidates (miR-144-5p and miR-29c-3p) showed significant differential expression (Supplementary Fig. 4A). ROC curves for miR-144-5p and miR-29c-3p are demonstrated in Supplementary Fig. 4B and revealed AUCs of 0.828 and 0.808, respectively. Given the low number of candidates found, it was decided not to proceed to validation with these candidates and focus on EV-miRNAs as our primary end-point.
Technical validation of EV-miR-200 family shows validity of differential expression
Following RNA isolation of 61 plasma EV samples (23 benign, 8 chronic pancreatitis (CP) and 30 PDAC), RT-qPCR was performed for the miR-200 family. In order to normalise RT-qPCR data, stable endogenous miRNAs were identified by applying NormFinder algorithms to the RNA-sequencing data. The geometric mean of stably expressed miR-23a, miR-26a and exogenous UniSp6 was defined as the EndoMean. Average Ct values were calculated for technical triplicate RT-qPCR assays of candidate miRNAs. Relative expression after normalisation gave rise to differential expressions analysis which confirmed upregulation in PDAC for each of the miR-200 family members, as shown in Fig. 5A. Logistic regression was performed to assess the diagnostic value of each miRNA individually, generating ROC curves and corresponding AUCs (Fig. 5B). The individual AUC for miR-200a was 0.783 (95%CI 0.668–0.897), for miR-200b 0.702 (95%CI 0.564–0.840), for miR-200c 0.728 (95%CI 0.600-0.856), for miR-141 0.765 (95%CI 0.644–0.885) and miR-429 0.668 (95%CI 0.531–0.804). In addition, RT-qPCR data of the EV-miR-200 family expression in plasma from healthy donors (n = 14) showed Ct values > 40, indicating very low expression, or absence of these miRNAs (Supplementary Table 4). These values were above the Ct value cut-off of 40. Therefore, we concentrated on distinguishing benign disease from malignant disease.
Validation of miR-200 family in plasma EV samples using RT-qPCR. (A) Expression of the EndoMean (the geometric mean of endogenous normalisers miR-23a, miR-26a and exogenous UniSp6) and the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) in plasma EV samples for the pairwise comparison: 30 PDAC vs. 31 benign. (B) Receiver operating curves with area under the curve (AUC) for each individual miR-200 family member is generated from logistic regression of the RT-qPCR expression. *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001
In combination, EV-miR-200 family showed an improved diagnostic accuracy with an AUC of 0.823 (95% CI 0.717–0.928; Fig. 6A). The calculated AUC for CA 19 − 9 in this cohort was 0.860 (95% CI 0.741 to 0.979; Fig. 6B), which was limited by missing values particularly in the benign population (17 out of 31; 54%). The addition of CA 19 − 9 to the model generated an AUC of 0.997 (95% CI 0.989-1.000; Fig. 6C).
Diagnostic value of the miR-200 family signature, which improved by addition of CA 19 − 9. Receiver operating characteristic curves and corresponding area under the curve values (AUCs) for (A) the miR-200 family (PDAC n = 30 vs. benign disease n = 31), (B) CA 19 − 9 (PDAC n = 28 vs. benign disease n = 14) and (C) the combination of the miR-200 family and CA 19 − 9 (PDAC n = 28 vs. benign disease n = 14). Patients with missing values for CA19-9 were not included in the analyses of (B) and (C). *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001
Clinical validation shows EV-miR-200 family is also upregulated in an independent cohort of patients
Differential expression of the EV-miR-200 family (miR-200a-3p, miR-200b-3p, miR200c-3p, miR-141-3p and miR-429) was confirmed in an independent validation cohort of 30 benign and 33 PDAC samples (Fig. 7A). Logistic regression analysis generated an AUC of 0.987 (95%CI 0.964-1.000; Fig. 7B, left) for diagnosing PDAC. When comparing CA19-9 measurements in 31 PDAC and 24 benign samples, CA19-9 demonstrated an AUC of 0.919 (95%CI 0.846–0.993) for predicting PDAC (Fig. 7B, middle). When combined with the EV-miR-200 family, it improved to an AUC of 1.00 (95%CI 1.00–1.00; Fig. 7B, right). Similar results were found when comparing benign disease with both PDAC and CCA, as illustrated in Supplementary Fig. 5, indicating that these miRNAs have potential to discriminate benign disease from both PDAC and CCA.
Confirmation of differential expression and accuracy of the EV-miR-200 family model in an independent cohort. (A) RT-qPCR results of EV-miR-200 family members in the clinical validation cohort for the pairwise comparison: PDAC (n = 33) vs. benign disease (n = 30). (B) ROC curves and corresponding AUCs for (left) the EV-miR-200 family signature (miR-200a-3p, miR-200b-3p, miR200c-3p, miR-141-3p and miR-429), (middle) CA 19 − 9, and (right) the combination of CA 19 − 9 and the EV-miR-200 family signature, for predicting PDAC. (C) Diagnostic accuracy of the EV-miR-200 family model for predicting PDAC (vs. benign) when applied to the clinical validation cohort, which consisted of benign (n = 30) and PDAC (n = 33) samples. AUC: area under the curve values; PDAC: pancreatic ductal adenocarcinoma; ROC: receiver operating characteristic. *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001
A novel plasma EV-miRNA Model for diagnosing PDAC in the clinical validation cohort
A plasma EV-miRNA dichotomous outcome (PDAC or benign) model was generated by logistic regression analysis using ∆Ct RT-qPCR miRNA expression data of the technical validation cohort. CA 19 − 9 was omitted from the models, as 54% of the patients with benign disease in the technical validation cohort did not have a recorded CA 19 − 9 level, and not all patients with malignant disease secrete CA 19 − 9. The miRNA model was then tested in the independent clinical validation cohort. When comparing PDAC (n = 30) and benign disease (n = 31) in the technical validation cohort, the EV-miR-200 family generated a diagnostic model (y = 3.559 + miR-200a-3p*0.3444 + miR-200a-3p*0.0757 + miR-200c-3p*0.0722 + miR-141*0.3049 + miR-429*-0.4012) with the most optimal cut-off at 0.5209. Application of this EV-miR-200 family model to the independent validation cohort showed a sensitivity of 100.0%, specificity of 88.2%, NPV of 100.0% and PPV of 87.9%, with an AUC of 0.970 (95%CI 0.925-1.000; p < 0.0001; Fig. 7C). When assessed in early-stage PDAC only (stage I/II, n = 25) vs. benign disease (n = 30), the model generated an AUC of 0.960 (95%CI 0.902-1.00; p < 0.0001; Supplementary Fig. 5A). In addition, in the subset of patients (n = 12) with T1 (i.e. tumour ≤ 2 cm in greatest dimension, 8th edition of the AJCC), or T2 (i.e. tumour > 2 cm in greatest dimension, but less than ≤ 4 cm), the model showed an AUC of 0.936 (95%CI 0.825-1.00; p < 0.0001); Supplementary Fig. 6B). For the comparison benign disease vs. PDAC plus CCA, this model showed a sensitivity of 100.0%, specificity of 83.3%, NPV of 100.0% and PPV of 90.8%, with an AUC of 0.984 (95%CI 0.961-1.000; p < 0.0001; Supplementary Fig. 5C).










Add Comment