Study setting
From May 1, 2023, to June 21, 2024, a single-center stepped wedge cluster randomized trial will be conducted within 35 units of Saint Marys Hospital and Methodist Hospital at Mayo Clinic in Rochester, Minnesota. The units include medical, surgical, trauma, and mixed ICUs and hospital floors that admit acute medical and surgical care patients as well as the emergency department (ED). The transitions between study phases will be initiated at 60-day intervals resulting in a 12-month study period. Mayo Clinic’s Institutional Review Board (IRB) approved the study as minimal risk (IRB-22-002974) and waived the requirement to obtain individual patient and clinician and provider consent due to the pragmatic nature of the design. The study has been registered on ClinicalTrials.gov NCT05860777.
Eligibility criteria
The recruitment and enrollment processes are broad and designed to simulate the use of the CT in practice. We will include those patients who will be admitted to the hospital or seen in the ED during the study period. Patients who have a NELP and complex care needs identified by the algorithm will be eligible. NELP will be identified and confirmed in several ways: through the algorithm-generated reports as well as using manual confirmation in the EHR by a human operator. Complexity will be identified using a palliative care score and supplemented by other predictors of complexity, such as length of stay, level of care, procedures, events, and clinical notes.
Patients will be excluded from the review if they are less than 18 years; do not have a language listed in EHR or any evidence of interpreter use; use sign language; are a confidential, unidentified, or non-verbal patient; or have an incomplete EHR. Patients who do not authorize the use of their EHR for research in accordance with the Minnesota state statute will be also excluded from the study [67].
Intervention
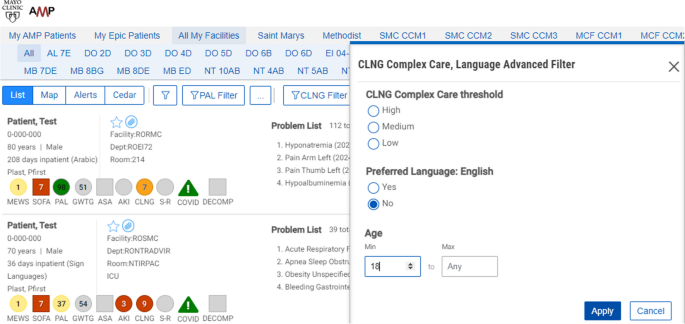
This study will be conducted using a workstation and software tool known as Control Tower. The CT is a web browser application that extracts medical data, processes the prediction algorithm, and presents the results through an ordered patient report list. See Fig. 1. More details can be found in Murphree et al.’s work [68].
In addition to the complexity score, additional data on language needed, age, other common risk scores, hospital unit/floor, and current length of stay are available and presented in the report to provide context for the calculated complexity score.
Patients with NELP in the intervention units will have complexity scores calculated within the Control Tower (≥ 9 points = severe complexity; 4–8 points = moderate complexity; < 4 points = mild complexity). Higher scores indicate increased complexity and need for in-person interpreter. Patients with NELP and calculated complexity scores are subsequently ranked from highest to lowest complexity score, as well as being color coded, with red indicating a score of 9, orange ≤ 9 ≥ 4, and yellow < 4. Newly admitted patients who are currently being evaluated by the algorithm have their scores labeled as grey. The complexity score is based on a machine learning palliative care score, supplemented by other predictors of complexity, including length of stay and level of care as well as events (clinical notes from teams and services involved in care, procedural notes, and diagnostic reports) (see Supplemental Table S1).
As part of the intervention, a CTO will engage with the language services coordinator responsible for coordinating, organizing, and providing in-person interpreters at study sites. The CTO will monitor the CT during regular weekdays starting early in the morning and generate a list once daily at 7 am. This list comprises adult patients with NELP and complex care needs, ordered by their complexity scores (from highest to lowest), and is assessed for any additional exclusion criteria in the development of the final list. Upon completing the screening process, the CTO will send the ordered list to the language services coordinator.
Units in the stepped wedge design will be randomized in their order for receiving treatment by a random sorting algorithm with a set seed for reproducibility. For our data pipeline, all dates for transition will be hard-coded so that the study participants enter the list when their admission corresponds to a study wedge. At each wedge transition, study staff will review the list to ensure that the program correctly switches patients over at the appropriate time. The number of patients on the list will vary and increase as we move forward with each stepped wedge of the trial every 60 days (adding 7 units to the intervention arm with each wedge). However, by working closely with language services leadership and operations managers, we believe this will be feasible even as the numbers in the intervention group grow. Rather than limiting the number of patients eligible to be included on the list sent to language services, it was agreed that should the list of patients in the intervention group become too large for disseminating secure chat messages and providing in-person interpreters, the organized list would help language services prioritize those patients with the highest complexity scores. This allows for the matching of patient need with the expected capacity of the language services team which is a pragmatic approach to balance effectiveness while avoiding interruptions to the usual workflow throughout the trial.
For those patients who are in the intervention arm, the language services coordinator will record if they have in-person interpreter for that particular language. Then, the language services coordinator will send a secure chat message via the EHR to advise the bedside nurse that the patient would benefit from an in-person interpreter and include contact information for how to reach language services. The secure chat message will be “Good morning. For an in-person interpreter, please contact Language Services at ext. X-XXXX with a date & time. We will do our best to meet the patient’s and medical team’s needs”. In contrast, the patients in the control units will continue to receive the regular standard of care, with patients potentially receiving an in-person interpreter or another interpretation modality following standard procedures by the primary healthcare team. Those patients in the control group will rely on clinician initiative to request language services.
Due to the potential disruption of using a new approach and tool such as a complexity score and CTO for promoting in-person interpreter use, we have communicated with multiple practice leaders and divisional and departmental committees. Additionally, we plan to send email communications to unit nurse managers prior to each stepped wedge roll out to prepare them.
In our study, 68% of the in-person interpreters are certified at the highest level for their respective languages by the Multiple National Certifying Organizations for Medical Interpreters. The remaining staff are in the process of certification, adhering to a strict code of ethics and standardized testing to ensure proficient language skills. Both study arms receive the same interpretation personnel and quality, but they follow different processes to access language services.
Outcomes
For all study outcomes, data will be collected through either the EHR or language services report list. The data outcomes will be abstracted during the patient hospitalization. The primary outcome is the number of patients with NELP and complex care needs who use an in-person interpreter during hospitalization in the units of interest as measured by language services daily report list. The secondary outcome will be time to first use of in-person interpreter—measured as time in hours and minutes from admission to in-person interpreter use as measured by the language services team electronic documentation system. The secure chat process measures including if sent, if responded to, and if not sent the reason such as no in-person interpreter on staff or available that day will be collected daily.
Participant timeline
The stepped wedge design involves allocating 35 floor and ICU units into a design matrix comprising 5 treatment wedges. Computer allocation will be used to generate the allocations prior to the start of the study. Each unit will cross over randomly from the control group (standard clinical practice and care) to the intervention group. Each wedge will span approximately 60 days, resulting in a study period of approximately 12 months unless specified otherwise. The initial step will entail a baseline period during which no intervention is administered, with all clusters receiving the intervention in the final step. Due to the pragmatic design of the trial, clinicians cannot be blinded to patient allocation to the intervention or control units, and we do not plan to implement blinding during the analysis as our endpoints are objective, and diffused roll-out would make this impossible [69]. This is most relevant if there are patient-reported outcomes. Patients will receive standard in-person interpreter services if requested and available in the hospital; however, those in the intervention group will differ in that clinicians will have been alerted to the patient’s need based on complexity score, CTO review and filter and secure chat sent by language services personnel. No additional patient data beyond the hospitalization will be collected, and there will be no follow-up visits. See Fig. 2.
Data analysis plan
Power statement
The proposed investigation will use a stepped wedge cluster randomized design with 12 clusters. Based on preliminary data, it is estimated that there are > 9000 inpatient admissions with NELP annually at our institution of which only 14 to 16% use the services of an in-person interpreter at some point during their hospitalization. For sample-size/statistical power considerations, we assume that the proportion of patients with NELP receiving in-person interpreter services at the start of our study period (while all patients are receiving usual care) will be 0.15. Although not all inpatients with NELP will meet study inclusion criteria, we believe that over the course of the year, the total number of inpatients who meet study inclusion criteria will be in the range of 500 to 700 for each of the 12 clusters. Stepped wedge cluster randomization trials typically have more statistical power than other cluster randomized designs when clusters are correlated, because each cluster is able to serve as its own control. Within-cluster correlation was introduced by using cluster-specific baseline rates (which ranged between 10 and 20%), and within-cluster correlation coefficients were not specified. Because of the complex nature of the design, we estimated statistical power using Monte Carlo simulation [70]. The Monte Carlo simulation for this paper was generated using the SAS software (Copyright © [2024] SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.) A logistic regression model was used for the simulation with the outcome being the use of in-person interpreter services. Simulations were created with the overall baseline percentage of patients receiving in-person interpreter services set at 15% (with cluster-specific baseline rates set between 10 and 20%) and included slight upward secular trend over time. Supplemental Table S2 presents the statistical power provided for detecting potential intervention effects corresponding to odds ratios ranging from 1.35 to 1.65 using 3 various sample size scenarios (500, 600, and 700 patients per cluster). Based on these simulations, the proposed investigation should have statistical power of > 80% to detect an odds ratio of 1.5 or greater.
We will generate overall and cluster-specific summaries of patient characteristics (sex, age, primary language, risk scores, etc.) using descriptive statistics, including mean ± SD for continuous variables with frequencies and percentages for nominal variables. The entire study population will be analyzed following an intention-to-treat (ITT) approach. The ITT analysis will include all patients in the intervention group regardless of whether the secure chat was sent and regardless of whether the clinician requested the service after receiving the secure chat. This principle will be extended to the cluster status in the event of transfers between intervention and control units. All missing data will be analyzed using complete case analysis. The primary outcome will be a binary variable representing any use of interpreter services during hospitalization. To assess the effects of the intervention, the primary outcome will be analyzed using a generalized linear mixed-effects model with variables: intervention approach and time period. The inpatient unit will be denoted as the random effect in the model to characterize the correlation among patients within the same cluster. An additional secondary outcome will be evaluated as the time from “intervention” to first use of an interpreter. Eligible patients are identified at 7 am daily, Monday to Friday. Time of intervention is calculated from the first time a patient is identified until documentation of first use of an interpreter. Patients may be eligible on multiple days and calculation of this outcome will start evaluation at 7 am on the first eligible day identified by the CTO. Those who never receive an interpreter will be assigned an adverse value (an arbitrarily large amount of time, reflecting an outcome worse than any observed time to interpreter). A generalized linear mixed-effects model with proportional odds link function will be used to evaluate this outcome to account for the stepped wedge cluster randomized study design including adjustment for time period and random effect for cluster. An alternative approach may consider reporting cumulative incidence estimates of time to interpreter by intervention group, with death or discharge a competing risk, which is functionally similar to the assignment of large arbitrary value for those without interpreter services. Patients who received interpreter services prior to identification by the CTO will be excluded from analyses as they do not meet inclusion criteria at “time zero” or baseline of the analysis. In all cases, the intervention effect will be summarized by reporting point estimates and corresponding 95% confidence intervals. Two-tailed p-values < 0.05 will be considered statistically significant [71, 72].
Data management
All data pertaining to study outcomes/model covariates and process measures will be collected through the following methods: (1) all input data received from the machine learning model will be logged each time the algorithm is called and stored in a study database; (2) study outcomes will be collected from the hospital EHR and language services daily report; (3) process measures (such as the number of secure chat messages sent and reasons not sent or in-person interpreter declined) will be gathered through the daily logs exchanged between the CTO and the language services team.
Data monitoring
The proposed intervention has been reviewed by the IRB and was determined to be a minimal risk study, so no data monitoring committee (DMC) will be created. Consequently, there will be no interim analyses or predefined stopping rules for prematurely ending the trial. The anticipated risks to patients in this study are expected to align with those encountered in routine clinical care. Ensuring patient safety will primarily rely on clinical staff adhering to established standards of care. Study logs will undergo bi-monthly audits for reporting purposes, but no decisions will be made based on the data to either stop or continue the trial.
The evaluation of study logs will involve several diverse personnel, the CTO, PI, study team statistical analyst, and IT personnel. The interpreter services personnel and CTO will monitor the algorithm while utilizing the tool to identify any potential errors affecting their workflow, such as missing complexity scores or inaccuracies in data elements or score components. The study team, PI, statistical expert, and IT support will review the logs to ensure complete field entries and mitigate omissions. The IT study team members will oversee the data pipelines to guarantee proper functionality across all data systems involved in score calculation. We will document all days when the pipeline fails to fire.
Confidentiality
Patient participation will occur through the utilization of hospital interpreter services with no additional contact or visits needed; therefore, we will follow the hospital’s policies and procedures for maintaining patient privacy and confidentiality with respect to data. For report purposes, we will use Agency for Healthcare Research and Quality (AHRQ) guidelines [73]. All results will be reported in aggregate with no cells size smaller than 10.
Dissemination policy
We anticipate given the novelty of our proposed work that we will have an opportunity to publish in the scientific literature regardless of outcome. Trial summary results will be submitted to ClinicalTrials.gov following the completion of the trial. The team have experience with publication, and we will follow all standard authorship and ethical requirements as specified in journals in which we publish. We anticipate that all authors of this protocol paper will also be authors of the subsequent outcome paper. We will use the Equator guidelines for reporting of pragmatic clinical trials to report our results [74]. Furthermore, here, we have included a checklist of recommended items to provide in clinical trial protocol [75] (Supplemental Table S3).







Add Comment