C.R infection causes gut microbiota alterations
C.R is a pathogen restricted to mice, and it shares 67% of its genes with human enteric pathogens [26]. C.R colonies were identified as pink colonies with narrow white trim on MacConkey agar plates (Fig. S1 A). Specific primers against the C.R EspF gene were applied for identification [27] (Fig. S1 B). The growth curve of C.R was established, and the bacterial concentration (CFU/mL) in the culture was calculated from the OD600 values (Fig. S1C-D).
Mice were orally administered with 4 × 108 CFU of C.R after fasting, and different parameters were recorded and analyzed according to the experimental scheme to confirm the effectiveness of C.R infection (Fig. 1A). A significant decrease in body weight was observed after C.R infection. At 9 days post infection (d.p.i.), the body weight of the mice was approximately 5% less than the initial weight (Fig. 1B). The fecal load of C.R was measured at 2-day intervals. The amount of C.R reached approximately 109 CFU/g in feces at 6 d.p.i and remained relatively constant until the end of monitoring (Fig. 1C). The fecal water content was monitored at 3-day intervals. Compared with uninfected mice with approximately 58% fecal water content, C.R-infected mice exhibited an increase in the percentage of water in the feces (63% at 3 d.p.i., 73% at 6 d.p.i., and 68% at 9 d.p.i.) and a maximum at 6 d.p.i. (C.R vs. control: p < 0.0001) (Fig. S2A). The food consumption of the animals was monitored at 9 d.p.i., and a notable decrease was observed in C.R-infected mice compared to the control mice (C.R vs. control: p = 0.0004) (Fig. S2B). The length of small intestinal transit was evaluated and there was a significant reduction compared to that in the control group (C.R vs. control: p = 0.0375) (Fig. S2C). In contrast, the spleen index of C.R-infected mice was greater than that of control group mice (C.R vs. control: p = 0.0017) (Fig. S2D), suggesting an increased immune response. Collectively, these data confirmed the effectiveness of C.R infection.
To investigate the effect of C.R challenge on the gut microbiota composition, we characterized changes in the gut microbiota composition in fecal samples from mice infected with C.R or vehicle solution (control group) at 9 d.p.i. according to high-throughput sequencing of the V3-V4 region of the 16 S rRNA genes. A significant reduction in α-Diversity was observed in the C.R-infected mice compared to the control mice based on the ACE index, an estimate of microbial community richness (C.R vs. control: p = 0.0016) (Fig. 1D). However, there were no differences between the groups in terms of the Shannon index, which is representative of microbial diversity (Fig. 1E). The extent of similarity of the gut microbial communities between the control group and the C.R group was measured using PCoA based on the weighted UniFrac distance and the Bray-Curtis dissimilarity to identify possible differences between the bacterial components in the gut microbiota. PCoA revealed that the gut microbiota of the C.R-infected mice was distinct from that of control mice (Fig. 1F-G). These findings indicated that the composition of the gut microbiota in C.R-infected mice was significantly different from that in control mice.
To further identify the critical bacteria affected by C.R colonization, we compared the relative abundances of microbes at various taxon levels between the groups. At the phylum level (Fig. 1H), the abundance of Firmicutes was significantly decreased in the C.R group compared to the control group (C.R vs. control: p < 0.0001) (Fig. S3A). In contrast, other phyla including Bacteroidetes, Verrucomicrobia, Proteobacteria and Epsilonbacteraeota, had higher abundances in the C.R group than the control group (C.R vs. control: p = 0.0420, p = 0.0219, p = 0.0048, p = 0.0087, respectively) (Fig. S3B-E). Moreover, the ratio of Bacteroidetes to Firmicutes was significantly higher in the C.R group than in the control group (C.R vs. control: p = 0.0067) (Fig. 1F). At the family level, as illustrated in the pie chart (Fig. 1I), the abundances of Bacteroidaceae, Enterobacteriaceae, Erysipelotrichaceae, Lachnospiraceae, and Prevotellaceae after the C.R challenge was increased compared to those in the control group, while the levels of Muribaculaceae, Lactobacillaceae and Clostridiaceae were reduced in the C.R group. As shown in Fig. 1J, overall significant differences in genera were detected between the groups. We further selected representative genera and utilized boxplots to display their detailed information (Fig. 1K-L and Fig. S3G-N). Compared to those in the control group, the relative abundances of Akkermansia (p = 0.0106) (Fig. 1K), Citrobacter (p = 0.0034) (Fig. S3G), Ruminococcus (p = 0.0035) (Fig. S3H), Helicobacter (p = 0.0087) (Fig. S3I), Coprobacillus (p = 0.0155) (Fig. S3G) and Parasutterella (p = 0.0117) (Fig. S3K) were significantly increased in the C.R group, whereas the abundances of the genera Lachnospiraceae (p = 0.0001) (Fig. 1L), Oscillibacter (p = 0.0219) (Fig. S3L), Clostridium (p = 0.0145) (Fig. S3M), and Roseburia (p = 0.0002) (Fig. S3N) were notably decreased in the C.R group. Collectively, these results indicated a significantly altered microbiota profile in C.R-infected mice.
C.R infection model establishment and analysis of the gut microbiome. (A) The scheme of C.R oral inoculation in the study. (B) The change in body weight relative to the initial weight of the mice was analyzed at the indicated time points after infection with C.R. n = 10–26. (C) Bacterial shedding in feces was monitored at 2-day intervals after infection with C.R. n = 10. (D-E) The analysis of α-Diversity based on 16 S rRNA sequencing predicted gut microbiota richness according to the ACE index (D) and diversity according to the Shannon index (E). (F-G) PCoA plots based on weighted UniFrac metrics of the gut microbiota where samples of mice from different groups are highlighted with different colors. PC1 and PC2 explained 49.44% and 14.56%, respectively, of the variance. PC1 and PC3 explain 49.44% and 7.67%, respectively, of the variance. The position and distance of the data points indicated the degree of similarity in terms of both the presence and relative abundance of the bacterial taxonomies. (H) Percentage stacking chart based on the Bray-Curtis distance analysis of the differences in the relative abundance of gut microbiota at the phylum level between the groups. (I) Differences in the relative abundance of gut microbiota at the family level between the groups. (J) Differences in the relative abundances of gut microbiota at the genus level between the groups. (K-L) Relative abundances of 2 significantly altered bacterial genera: Akkermansia (K), and Lachnospiraceae (L). n = 8–10. **p < 0.01, ***p < 0.001, ****p < 0.0001
C.R colonization damages the intestinal barrier and induces intestinal inflammation
C.R is located mainly in the distal part of the colon and cecum during the steady-state phase [28]. To explore the destructive effect of C.R colonization on the intestine, the morphology and pathology of the 0.5-cm terminal colon were evaluated at 9 days after infection. Morphologically, there was an evident lesion indicated by the swelling of the distal colon of C.R-infected mice, while colons of the control mice were normal and filled with feces (Fig. 2A). Simultaneously, the C.R-infected mice exhibited a significant decrease in colon length (C.R vs. control: p < 0.0001) (Fig. 2B). To better define the basis of these differences, the terminal colon was examined by H&E staining. The colon mucosa of the control mice displayed an intact epithelium and no overt hyperplasia, while C.R-infected mice showed significant damage, as demonstrated by widespread epithelial cell sloughing, crypt hyperplasia, submucosal edema and extensive polymorphonuclear cell infiltration (Fig. 2C). Correspondingly, when the pathology was scored, the score of tissue damage in the colon of C.R-infected mice was significantly higher than that of the untreated mice (C.R vs. control: p = 0.0024) (Fig. 2D). Goblet cell depletion was also detected in the colon tissue (Fig. S4).
At the molecular level, intestinal barrier dysfunction in the colon was evaluated via immunofluorescence staining for the tight junction proteins zonula occludens-1 (ZO-1), Occludin, and Claudin-1 (Fig. 2E). The pattern showed continuous, robust expression of ZO-1 at the epithelial lining of the control mice while the C.R-infected mice exhibited a disrupted, discontinuous structure of ZO-1 (Fig. 2E). Semiquantitative analysis of the staining using an integrity scoring scale demonstrated a notable decrease after C.R infection (C.R vs. control: p = 0.0296) (Fig. 2F). Microscopic analysis revealed a stronger Occludin staining in the epithelial lining of the control group (Fig. 2E). The integrity scores for Occludin expression were significantly lower after C.R infection (C.R vs. control: p = 0.0003) (Fig. 2G). Microscopic visualization revealed that Claudin-1 was clearly expressed in the colonic epithelial lining of the control group, and Claudin-1 expression was significantly impacted by C.R infection (Fig. 2E). The analysis also revealed significantly lower expression of Claudin-1 in C.R-infected mice than in control mice (C.R vs. control: p = 0.0093) (Fig. 2H). Consistently, the mRNA expression of multiple tight junction-related genes in the colon was significantly decreased in the C.R-infected group (C.R vs. control: Ocln, p = 0.0004; Cldn3, p = 0.0146; Cldn4, p = 0.0062; Cldn7, p = 0.0356; Cldn8, p = 0.2302; Cldn12, p < 0.0001; Tjp1, p = 0.0022; Tjp2, p = 0.0014; Tjp3, p = 0.0176) (Fig. 2I). In addition, an intestinal permeability assay was conducted in vivo through oral delivery of 4 KDa FITC-dextran (FD4), and a greater increase in FD4 in the serum represented elevated gut permeability. The results showed that there was a remarkable increase in FD4 intensity after C.R challenge (C.R vs. control: p = 0.0065) (Fig. 2J). All these data suggested that C.R colonization caused intestinal barrier impairment.
Immunofluorescence staining revealed a different pattern of GFAP+ enteric glial reactivity in the myenteric plexuses of the colon tissue at 9 days post infection (Fig. 2L). GFAP intensity in the lamina propria of C.R-infected mice was significantly higher than that in the control mice (C.R vs. control: p = 0.0005) (Fig. 2M). In addition, the colon of the C.R-infected mice showed a considerably increased mRNA expression of proinflammatory factors, including IL-1β (p = 0.0237), IL-6 (p < 0.0001), IL-12 (p = 0.0004), iNOS (p = 0.0011), and the anti-inflammatory factor IL-4 (p = 0.0005), but decreased mRNA expression of the TNF-α (p = 0.1587) compared to the colon of control mice (Fig. 2K). Fecal calprotectin has been used as an indicator of intestinal inflammation [29]. ELISA data showed that the level of calprotectin in the fecal pellets of C.R-infected mice was significantly higher than that in the control mice (C.R vs. control: p < 0.0001) (Fig. 2N). Taken together, these data demonstrated that C.R colonization induced intestinal inflammation.
The intestinal barrier damage and intestinal inflammation induced by C.R infection. (A) The colonic morphology of the control and C.R-infected mice. The arrows indicate lesion sites in the distal colon. (B) The colon length. n = 5. (C) H&E staining of colons. Scale bar, 200 μm (top) and 20 μm (bottom). (D) Histopathological scoring of colonic tissue. n = 4–5. (E) Representative immunofluorescence images of the tight junction proteins ZO-1, Occludin, and Claudin 1 in colon tissue. Scale bar, 100 μm. (F-H) An arbitrary scale of 0–3 (0 = no expression, 3 = continuous normal expression of the barrier) was used to assess barrier integrity. (F) Integrity scoring data for ZO-1. (G) Integrity scoring data for Occludin. (H) Integrity scoring data for Claudin 1. n = 4. (I) The expression of nine tight junction proteins in colonic tissue was analyzed by qPCR. n = 4–6. (J) The serum concentrations of FD4. n = 5. (K) The levels of six inflammatory factors in colonic tissue were analyzed by qPCR. n = 3–5. (L) Representative images of GFAP+ cells in the myenteric plexuses. Normal: scale bar,100 μm; Magnification: scale bar, 10 μm. The triangle arrow indicates GFAP+ cells in the myenteric plexuses. (M) Optical density analysis of GFAP expression in the myenteric plexuses. n = 4–5. (N) The level of calprotectin in feces was analyzed by ELISA. n = 7. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
C.R infection regulates the metabolism of short-chain fatty acids in the gut and neurotransmitters in the brain
Mounting evidence has demonstrated that SCFAs are involved in the regulation of intestinal inflammation [30]. To explore the potential mechanisms of action of C.R challenge in gut inflammation, the fresh fecal samples were collected for the evaluation of SCFAs by HPLC. The results showed that compared to that in the control mice, the concentration of propionic acid (C.R vs. control: p = 0.0034) (Fig. 3B) and butyric acid (C.R vs. control: p = 0.0004) (Fig. 3C) exhibited a notable decrease while the level of acetic acid (Fig. 3A) remained unchanged in the C.R-infected mice. Moreover, the SCFA receptors GPR109a and GPR43 were detected in the colon. The data showed that there was lower expression of GPR109a and GPR43 in the C.R group than in the control group (C.R vs. control: p = 0.0218 and p = 0.0396, respectively) (Fig. 3D-E). In brief, these data suggested that C.R infection affected SCFA metabolism in the gut.
Gut microbiota alterations is linked to the disturbance of neurotransmitter metabolism [31]. To verify the effect after C.R infection, the metabolism of tyrosine (Tyr) and tryptophan (Trp) in the striatum was assessed at 9 days after C.R colonization. Surprisingly, the DA level was decreased (C.R vs. control: p = 0.0183) (Fig. 3F), while the levels of DOPAC (C.R vs. control: p = 0.0496) (Fig. 3G), HVA (C.R vs. control: p = 0.0445) (Fig. 3H) were markedly increased in the Tyr metabolism pathway.
To further explore the underlying mechanism, the step limiting enzyme tyrosine hydroxylase (TH), MAOA, MAOB, catechol-O-methyltransferase (COMT) and dopamine transporter (DAT) were investigated (Fig. 3N). We found that the expression of TH, DAT and COMT did not change (C.R vs. control) (Fig. 3Q, O and S), while MAOA (C.R vs. control: p = 0.0142) (Fig. 3P) and MAOB (C.R vs. control: p = 0.0142) (Fig. 3R) expression was markedly increased. In the Trp metabolism pathway, the levels of the metabolites 5-HT and 5-HIAA in C.R-infected mice were markedly lower than those in the control mice (C.R vs. control: p = 0.0021 and p = 0.0094, respectively) (Fig. 3L and K). Additionally, the ratios of DOPAC/DA (C.R vs. control: p = 0.0271) (Fig. 3I), and HVA/DA (C.R vs. control: p = 0.0368) (Fig. 3J), and 5-HIAA/5-HT (C.R vs. control: p = 0.0131) (Fig. 3M) were obviously increased, indicating increased turnover of DA and 5-HT in C.R infection. In addition, similar trends in Tyr and Trp metabolism were also observed in the female mice (Fig. S5).
To further explore whether there was a continuous and long-term effect on neurotransmitter metabolism in the striatum, the animals were sacrificed at 9, 15, 21 and 30 days after C.R challenge. The results showed that there was a remarkable decrease in DA in the C.R-infected group in comparison with the control group, with the lowest level being observed at 15 d.p.i. (Fig. 3T). In contrary, the levels of DOPAC and HVA increased during C.R infection compared to the control group, with the peaks levels of both metabolites appearing at 21 d.p.i. (Fig. 3U and V). At the end of monitoring, the DOPAC and HVA levels returned to baseline (Fig. 3U and V). The levels of 5-HT and its metabolite 5-HIAA in the C.R group were lower than those in the control group. After C.R colonization for 15 days, 5-HT levels were gradually returned to normal (Fig. 3W), while 5-HIAA levels showed a continuous decline (Fig. 3X).
C.R infection caused metabolic abnormalities in SCFAs in the gut and in neurotransmitters in the striatum. (A-C) The levels of acetic acid (A), propionic acid (B), and butyric acid (C) in feces at 9 days post infection. n = 6. (D, E) The mRNA levels of GPR109a(D) and GPR43(E) in colonic tissue. n = 5–7. (F-M) The metabolism of DA and 5-HT. DA (F), DOPAC (G), HVA (H), DOPAC/DA (I), HVA/DA (J), 5-HIAA (K), 5-HT(L), and 5-HIAA/5-HT (M). n = 5–6. (N) Western blotting showing striatal levels of the DAT, MAOA, TH, MAOB and COMT proteins. β-actin served as the loading control. (O-S) Quantification of the relative expression levels of the DAT (O), MAOA (P), TH (Q), MAOB (R) and COMT (S) proteins. n = 3–6. (T-X) The metabolism of DA and 5-HT at 9, 15, 21, and 30 days post infection. DA (T), DOPAC (U), HVA (V), 5-HT(W), 5-HIAA (X). n = 3–10. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
To further validate the effective components of C.R gavage, the Luria Broth (LB), supernatant and precipitate of C.R culture medium were given by gavage according to the manufacturer’s instructions (Fig. S6A). The levels of neurotransmitters and metabolites (DA, DOPAC, HVA, 5-HT and 5-HIAA) in the striatum were evaluated by HPLC after 12 days of continuous treatment. Compared with those in LB-treated mice, the five substances remained unchanged in mice treated with the supernatant or the precipitate (Fig. S6B). Moreover, there was no change in the expression of the TH and MAOB proteins after supernatant and precipitate treatment compared with that in the LB-treated group (Fig. S6C-E). Taken together, these results indicated that only living C.R delivery disturbs neurotransmitter metabolism.
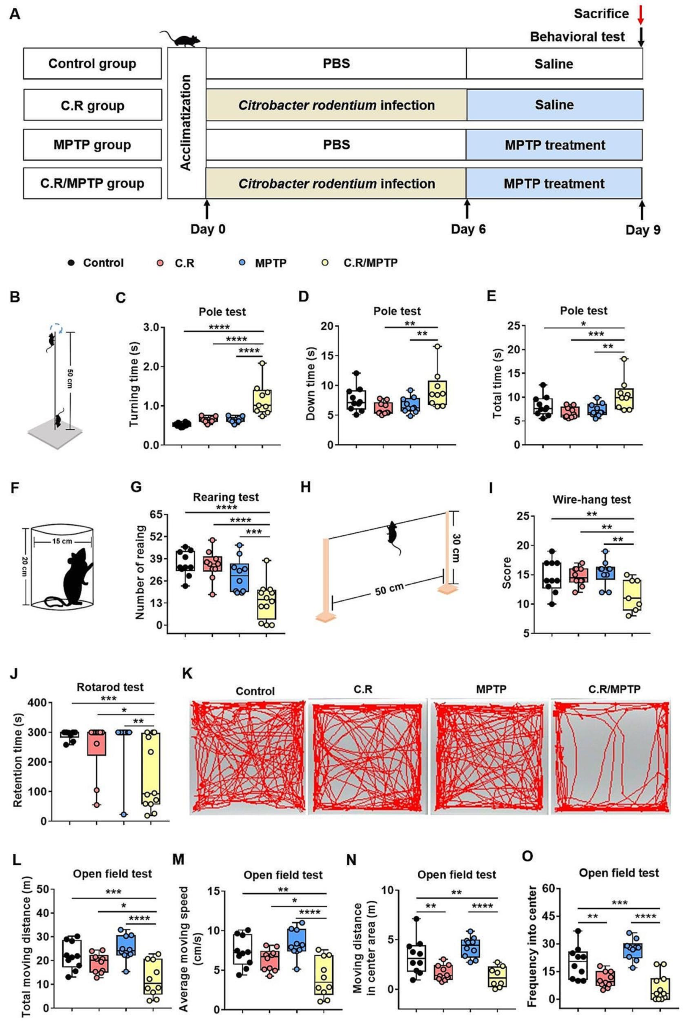
C.R in combination with MPTP challenge causes motor deficits
As mentioned in the literature, imbalances in neurotransmitter metabolism and the gut microbiota are causal factors of PD pathogeny [32]. To evaluate the effects of intestinal disorders on PD pathogenesis, we established an infection mouse model by orally giving mice C.R once. At 6 days post inoculation, the C.R-challenged mice were administrated 40 mg/kg MPTP or vehicle (Fig. 4A). At 9 d.p.i. (3 days after MPTP administration), various behavioral tests, including the pole test, the rotarod test, the wire-hanging test and the rearing test, were performed to assess motor function. Overall, there were no significant behavioral changes in these mice, regardless of treatment with C.R or MPTP alone compared to the control group (Fig. 4B-J). However, the mice administered C.R and MPTP combined exhibited significant motor disorders, including delayed initiation of movement, as indicated by a longer turning time (C.R/MPTP vs. control: p < 0.0001) (Fig. 4C), more time spent climbing down the pole (C.R/MPTP vs. control: p = 0.0077) (Fig. 4D) and the whole climbing process in the pole rest (C.R/MPTP vs. control: p = 0.0024) (Fig. 4E), the motor deficit characterized by less standing time in the rearing test (C.R/MPTP vs. control: p = 0.0006) (Fig. 4G), poorer muscle force manifested as a lower score in the wire-hanging test (C.R/MPTP vs. control: p = 0.0020) (Fig. 4I), and decreased retention time on the rod (C.R/MPTP vs. control: p = 0.0034) (Fig. 4J) in the rotarod test.
In addition, the open field test (OFT) was also performed to evaluate locomotion and emotion. The data showed that there were no marked changes in distances traveled or the speed of the mice treated with C.R alone or MPTP alone compared with those of the control mice (Fig. 4K-M) in the OFT. C.R in combination with MPTP challenge prominently caused less movement (C.R/MPTP vs. control: p < 0.0001) (Fig. 4L) and bradykinesia (C.R/MPTP vs. control: p < 0.0001) (Fig. 4M). C.R alone or C.R and MPTP combined challenge led to shorter distances in the central area (C.R vs. control: p = 0.0045, C.R/MPTP vs. control: p < 0.0001) (Fig. 4N) and a lower frequency in the central area (C.R vs. control: p = 0.0036, C.R/MPTP vs. control: p < 0.0001) (Fig. 4O) than vehicle. The OFT results suggested that C.R infection also participated in emotional regulation. Taken together, these data suggested that C.R and MPTP induced motor impairments and slight anxiety-like behavior.
The combination of C.R and MPTP caused movement impairments in PD mice. (A) The experimental design in the current study. (B-E) Behavioral changes were assessed in the pole test (B), including the time to turning (C), time to reach the bottom (D) and the total time (E). (F-G) Behavioral changes were assessed in the rearing test (F) and number of rearing in a 3-minute period (G). (H-I) Behavioral changes were assessed via the wire-hanging test (H) and the total score over 3 min (I). (J) Behavioral changes were assessed in the rotarod test and the retention time on the rod at speed of 32 rpm was recorded. (K-O) Behavioral changes were assessed in the open field test; representative traces (K), total moving distance (L), average moving speed (M), moving distance in the center area (N), and frequency in the center (O) are shown. n = 7–12. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
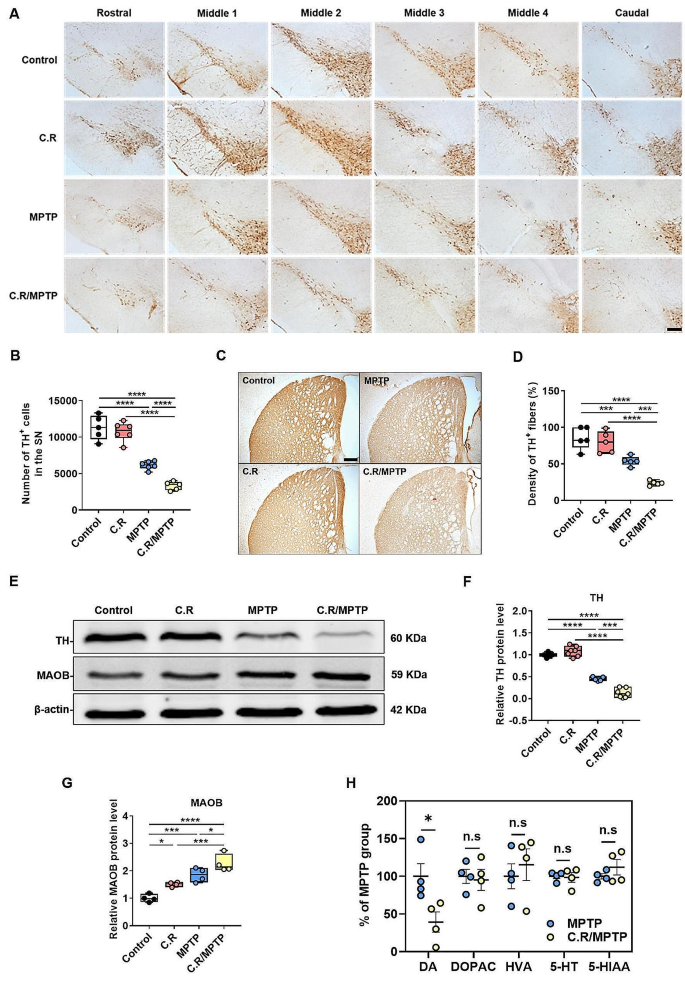
C.R infection in combination with MPTP exacerbates the loss of TH-positive dopaminergic neurons and TH protein expression in the brain
Given that dopaminergic neuronal loss in the nigrostriatal system is the main pathological characteristic of PD, we examined whether C.R infection also has a destructive effect on the dopaminergic system in the SN and the striatum. Tyrosine hydroxylase (TH) is an enzyme involved in DA synthesis; therefore, TH+ cells are dopaminergic neurons. Representative images of immunohistochemical staining of the SN rostral to caudal in the coronal section are shown in Fig. 5A. By stereological counting analysis of the SN, we found that the number of TH+ cells decreased by nearly 50% after MPTP exposure alone (MPTP vs. control: p < 0.0001), while no significant difference was detected in mice with C.R infection alone compared to the control mice (Fig. 5B). However, C.R in combination with MPTP significantly exacerbated the loss of dopaminergic neurons in PD mice (C.R/MPTP vs. MPTP: p = 0.0006) (Fig. 5B). Consistent with this observation, immunostaining and western blotting of the striatum also revealed marked decreases in the TH+ fiber (MPTP vs. control: p = 0.0004) and TH protein (MPTP vs. control: p < 0.0001) levels in the MPTP group compared to those in the control group, while there was no change in mice challenged with C.R alone compared to the control mice (Fig. 5C-D and E-F, respectively). Nevertheless, there was a more severe decrease in TH+ fibers and TH protein expression in the C.R/MPTP group compared to the MPTP-challenged group (C.R/MPTP vs. MPTP: TH+ fibers, p = 0.0006; TH protein levels, p < 0.0001) (Fig. 5C-D and E-F, respectively). MAOB is responsible for the enzymatic degradation of dopamine at axon terminals, often showing a negative correlation with TH expression. There were an elevated protein levels of striatal MAOB in only C.R exposed or only MPTP exposed mice compared to the control mice (C.R vs. control: p = 0.0392; MPTP vs. control: p = 0.0012) (Fig. 5E-G). However, C.R in combination with MPTP significantly increased MAOB expression in comparison with MPTP alone (C.R/MPTP vs. MPTP: p = 0.0250) (Fig. 5E-G). Moreover, the level of DA in the striatum was significantly decreased in the C.R/MPTP group compared to the MPTP group (C.R/MPTP vs. MPTP: p = 0.0300) (Fig. 5H), while the levels of the DA metabolites DOPAC and HVA were not different between the groups (Fig. 5H). The levels of 5-HT and its metabolite 5-HIAA also did not differ between the MPTP group and the C.R/MPTP group (Fig. 5H).Taken together, these data confirm that peripheral C.R infection aggravated the nigrostriatal dopaminergic system lesions.
C.R infection aggravated MPTP-induced nigrostriatal system lesions. (A) Immunohistochemical staining showing TH+ cells in the SN. (B) Results of stereological counting of TH+ cells in the SN. Scale bar, 100 μm. n = 5–6. (C) Immunohistochemical staining showing striatal TH+ nerve fibers. Scale bar, 200 μm. (D) Densitometric analysis of the relative optical density of the staining. n = 5. (E) Western blotting showing the striatal levels of the TH and MAOB proteins. β-Actin served as the loading control. (F, G) Quantification of the relative protein expression levels of TH and MAOB. TH: n = 5–7; MAOB: n = 3–4. (H) The levels of DA, DOPAC, HVA, 5-HT and 5-HIAA in the striatum. n = 4. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Activation of microglia in the SN and the striatum is exacerbated after C.R and MPTP combination treatment
Iba1 is a marker of microglia. We first used Western blotting analysis to evaluate the expression of Iba1 in the striatum. The results demonstrated that the level of Iba1 protein was greater in the MPTP group than in the control group (MPTP vs. control: p = 0.0146) (Fig. 6A-B), and the C.R treatment alone did not affect Iba1 expression (Fig. 6A-B). In the C.R/MPTP group, the Iba1 protein level was significantly increased compared to that in the MPTP group (C.R/MPTP vs. MPTP: p = 0.0164) (Fig. 6A-B).
Next, we evaluated the number and morphology of microglia within the striatum via immunohistochemical assays. The results showed that Iba1+ cells in the control group or the C.R group were characterized by a small number of highly-ramified morphologies (Fig. 6C). After MPTP intoxication or C.R and MPTP combined treatment, numerous activated Iba1+ microglia with stouter cell processes and a round shape were observed (Fig. 6C). In terms of quantity, the number of Iba1+ cells were markedly greater in MPTP-challenged mice than the control mice (MPTP vs. control: p = 0.0015) (Fig. 6C-D). Conversely, there was no impact on mice challenged with C.R alone (Fig. 6C-D). However, in the C.R/MPTP group, the number of Iba1-labeled microglia was significantly higher than that in the MPTP group (C.R/MPTP vs. MPTP: p = 0.0135) (Fig. 6C-D).
Furthermore, the morphology of the microglia was analyzed. There was no obvious difference between the C.R group and the control group (Fig. 6E-G). Compared with those in the control group, the soma of Iba1-labeled microglia in the MPTP-treated group were enlarged (MPTP vs. control: p = 0.0046) (Fig. 6E), the summed process length (MPTP vs. control: p < 0.0001) (Fig. 6F) and total number of endpoints (MPTP vs. control: p < 0.0001) (Fig. 6G) were decreased. Strikingly, in the C.R/MPTP group, activated microglia exhibited a larger soma area (MPTP vs. control: p = 0.0002) (Fig. 6E), a shorter summed process length (MPTP vs. control: p = 0.0034) (Fig. 6F) and fewer endpoints of microglia (MPTP vs. control: p = 0.0010) than did the MPTP group (Fig. 6G).
Similar results were obtained when microglia in the SN were analyzed. In the present study, we performed immunofluorescence staining of the SN region to detect the activation of glial cells, using Iba1 as a marker of microglia (Fig. 6H). The number of Iba1+ cells was significantly greater in the MPTP group than in the control group (MPTP vs. control: p < 0.0001) (Fig. 6J). No effect on Iba1+ cells was observed in mice infected with C.R alone (Fig. 6J). However, more Iba1+ cells were found in mice challenged with C.R and MPTP than in mice challenged with MPTP alone (C.R/MPTP vs. MPTP: p = 0.0033) (Fig. 6J).
To evaluate the classic M1 phenotype of microglia in the SN, the expression of CD16/32, a typical marker of the M1 activation, was analyzed. The data showed that there were no obvious CD16/32-labeled microglia in the SN in the control or C.R-infected mice (Fig. 6I and K). There were more CD16/32-labeled cells in the MPTP group than in the control group (MPTP vs. control: p < 0.0001) (Fig. 6I and K). In the C.R/MPTP group, the number of CD16/32-labeled cells was greater than that in the MPTP group (C.R/MPTP vs. MPTP: p < 0.0001) (Fig. 6I and K).Collectively, these results illustrated that peripheral C.R infection significantly exacerbated MPTP-induced microglial activation in the nigrostriatal pathway.
The C.R challenge exacerbated MPTP-induced microglial activation in the striatum and the SN. (A) Western blotting showing striatal levels of Iba1 proteins. β-Actin served as the loading control. (B) Quantification of the relative protein expression levels of Iba1. n = 4–5. (C) Immunohistochemical staining showing Iba1+ microglial cells in the striatum. Scale bar, 40×: 20 μm, 100×: 5 μm. (D) The number of Iba1+ cells. n = 5. (E-G) Morphological analysis of Iba1+ cells, the soma area (E), the summed process length (F), and the endpoints of microglia (G). n = 5. (H) Immunofluorescence staining of TH (green), Iba1 (red) and DAPI (blue) in the SN. Scale bar, 100 μm. (I) Immunofluorescence staining of Iba1 (green), CD16/32 (red) and DAPI (blue) in the SN. Scale bar, 200 μm. (J) The numbers of Iba1+ cells in the SN. (K) The ratio of the number of CD16/32+ and Iba1+ cells to the total number of Iba1+ cells. n = 4–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Activation of astrocytes in the SN and striatum is exacerbated after C.R and MPTP combined challenge
Astrocytes, another important type of glial cell, play critical roles in homeostasis in the brain. We analyzed astrocyte activation by assessing GFAP expression. Western blotting data showed revealed greater levels of GFAP protein in the striata of MPTP-delivered mice than in those of the control mice (MPTP vs. control: p = 0.0002) (Fig. 7A, B), while C.R alone had no effect on GFAP expression (Fig. 7A, B). Strikingly, the protein level of GFAP was dramatically higher in the C.R/MPTP group than the MPTP group (C.R/MPTP vs. MPTP: p = 0.0001) (Fig. 7A, B). Moreover, the number of GFAP+ cells in the striatum was increased, consistent with the protein expression data (Fig. 7C, D). Specifically, there were more GFAP+ cells in the C.R/MPTP group than the MPTP group (C.R/MPTP vs. MPTP: p = 0.0008) (Fig. 7D). In addition, similar results were acquired in the SN by immunofluorescence staining and cell counting (Fig. 7E and G).
To evaluate the neurotoxic effects on the A1 phenotype of astrocytes in the SN, we analyzed C3, a marker of A1 activation. There were more C3+ cells in the MPTP group than in the control group (MPTP vs. control: p = 0.0019) (Fig. 7F and H). In the C.R/MPTP group, the number of C3-labeled cells was markedly higher than that in the MPTP group (C.R/MPTP vs. MPTP: p < 0.0001) (Fig. 7F and H). Collectively, these results demonstrated that the C.R challenge dramatically exacerbated neurotoxicity-mediated astrocyte activation in the nigrostriatal pathway.
The combination of C.R and MPTP exacerbated neurotoxicity-mediated astrocyte activation in the nigrostriatal pathway at 9 days post infection. (A) Western blotting showing striatal levels of GFAP protein. β-Actin served as the loading control. (B) Quantification of the relative expression levels of GFAP protein. n = 5. (C) Immunohistochemical staining showing GFAP in the striatum. Scale bar, 20 μm. (D) The number of GFAP+ cells in the striatum. n = 4–5. (E) Immunofluorescence staining of TH (green), GFAP (red) and DAPI (blue) in the SN. Scale bar, 200 μm. (F) Immunofluorescence staining of GFAP (green), C3 (red) and DAPI (blue) in the SN. Scale bar, 50 μm. (G) Number of GFAP+ astrocytes in the SN. (H) The ratio of the number of C3+ and GFAP+ cells to the total number of GFAP+ cells. n = 3–5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
C.R administration activates the TLR4 signaling pathway in the colon and the nigrostriatal pathway in the current model system
C.R is gram-negative bacteria that contain LPS on their membrane surface. TLR4 can recognize and respond to LPS, and its downstream pathways dominate the microbiota-gut-brain axis when gastrointestinal infections or systemic bacterial infections occur [33]. To examine the activation status of the TLR4 pathway in the colon and the nigrostriatal pathway, qPCR, western blotting, and immunofluorescence staining were performed.
In the colon, the mRNA level of TLR4 was higher in the C.R (C.R vs. control: p = 0.0033) and C.R/MPTP groups (C.R/MPTP vs. control: p = 0.0104) (Fig. 8A) than the control group. As revealed by Western blotting, except for the MPTP group, the C.R and C.R/MPTP groups showed a significantly greater expression of TLR4 and NF-κB p65 protein expression than the control group (C.R vs. control: p = 0.0328 and p = 0.0333, C.R/MPTP vs. control: p = 0.0010 and p = 0.0002, respectively) (Fig. 8C and D); while the expression of TLR4 and NF-κB p65 in the C.R group was lower than that in the C.R/MPTP group (C.R vs. C.R/MPTP: p = 0.0295 and p = 0.0371, respectively) (Fig. 8C and D). In addition, the immunofluorescence staining results revealed that the numbers of TLR4+ cells in the colon were notably higher in the C.R and the C.R/MPTP groups than to the control group (C.R vs. control: p = 0.0001, C.R/MPTP vs. control: p = 0.0005), whereas the number of TLR4+ cells in the colon of MPTP-injected mice was similar to that in colon of control mice (Fig. 8E and F). In addition, we also measured the levels of IL-6 in mouse serum. The ELISA results demonstrated that there was a trend toward an increase in the MPTP group compared to the control group (Fig. 8G). However, the C.R/MPTP group showed greater levels of IL-6 than did the MPTP group (C.R/MPTP vs. MPTP: p = 0.0035) (Fig. 8G).
The TLR4 signaling pathway was activated in the colon after the C.R challenge. (A) The mRNA level of TLR4 in the colon. n = 4. (B) Western blotting showing colonic levels of the TLR4 and NF-κB p65 proteins. β-Actin served as the loading control. (C-D) Quantification of the relative protein expression levels of TLR4 and NF-κB p65. n = 4–6. (E) Immunohistochemical staining showing TLR4 in the colon. Scale bar, 50 μm. (F) The number of TLR4+ cells in the colon in per field. n = 4. (G) The IL-6 protein level in the serum was analyzed by ELISA. n = 5. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Similarly, we measured TLR4 expression in the nigrostriatal pathway. In the striatum, the levels of TLR4 (C.R/MPTP vs. control: p = 0.0125) and the ratio of NF-κB pp65/p65 (C.R/MPTP vs. control: p = 0.0191) were higher in the C.R/MPTP group than the control group (Fig. 9A-C). The similar trends in TLR4 expression in the SN were confirmed by immunofluorescence staining (MPTP vs. control: p = 0.0018, C.R/MPTP vs. C.R: p = 0.0121, C.R/MPTP vs. control: p = 0.0078) (Fig. 9D-E). All the evidence supported that combined C.R and MPTP challenge activated the TLR4-NF-κB signaling pathway.
The TLR4-NF-κB signaling pathway was activated in the nigrostriatal pathway in our model system. (A) Western blotting showing striatal levels of TLR4, NF-κB p65, and NF-κB pp65 proteins. β-Actin served as the loading control. (B-C) Quantification of the relative expression levels of TLR4 and the ratio of NF-κB pp65/p65 proteins. n = 5. (D) Immunohistochemical staining showing TLR4 in the SN. Scale bar, 20 μm. (E) The number of TLR4+ cells in the SN in per field. n = 4. *p < 0.05, **p < 0.01
C.R and MPTP combined treatment causes or exacerbates neuroinflammation and alters the gut microbiota composition
To confirm the inflammatory process among the different groups, we first measured the corresponding mRNA expression of inflammatory cytokines in the striatum. The results displayed a remarkable upregulation in the MPTP group compared to the control group (MPTP vs. control: IL-6, p = 0.0131; IL-1β, p = 0.0182; Aif1, p = 0.0132; S100b, p = 0.0132) (Fig. 10A). However, the levels in the C.R/MPTP group were even higher than those in the MPTP group (C.R/MPTP vs. MPTP: IL-6, p = 0.0001; IFN-γ, p = 0.0055; S100b, p = 0.0379; iNOS, p = 0.0486; CD86, p = 0.0039; CD68, p = 0.0047; COX2, p = 0.0293) (Fig. 10A and B). However, the mRNA level of BDNF was lower in the C.R-, MPTP- and C.R/MPTP groups than the control group (C.R vs. control: p = 0.0051, MPTP vs. control: p = 0.0361, C.R/MPTP vs. control: p = 0.0003) (Fig. S7). Importantly, there was much lower expression in the C.R/MPTP group than the MPTP group (C.R/MPTP vs. MPTP: p = 0.0248) (Fig. S7). In addition, the expression of one representative proinflammatory factor (COX2) was measured by western blotting. COX2 protein expression was notably greater in the MPTP group than in the control group (MPTP vs. control: p = 0.0241). Compared to that in the MPTP group, the level of COX2 in the C.R/MPTP group was further increased (C.R/MPTP vs. MPTP: p = 0.0417), while the COX2 level in the C.R group remained unchanged (Fig. 10C). Additionally, the TLR4 antagonist TAK242 was used to prevent the activation of TLR4, and we found that TAK242 had no effect on striatal DA metabolism (Fig.S8).
To investigate the effect of C.R challenge on MPTP-induced PD pathology, we compared the composition of the gut microbiota between the groups receiving MPTP and C.R plus MPTP treatment at 9 d.p.i using 16 S rRNA sequence analysis. We observed a significant increase in α-Diversity, as assessed by the ACE index, in the C.R. plus MPTP-treated group compared to the MPTP group (MPTP vs. C.R/MPTP: p = 0.0109) (Fig. S9A). However, no significant differences were found in the Shannon index between the groups (Fig. S9B). PCoA and Bray-Curtis dissimilarity revealed a distinct gut microbiota composition for C.R/MPTP-treated mice compared to MPTP-treated mice (Fig. S9C-D). Furthermore, to identify the specific bacteria impacted by C.R colonization in MPTP-treated mice, we conducted a comparative analysis of microbial relative abundances at various taxonomic levels. At the phylum level (Fig. S9E), the abundance of Bacteroidota and Firmicutes showed a significant decline in the C.R/MPTP group compared to the C.R group, whereas Proteobacteria, in contrast, exhibited a higher abundance in the C.R/MPTP group than the MPTP group (Fig. S9E). At the family level, as illustrated in the pie chart (Fig. S9F), the abundances of Citrobacter, Parabacteroides, Marvinbryantia, and Blautia were higher in the C.R/MPTP group than the MPTP group, while the levels of Muribaculaceae, Lactobacillus, and Lachnospiraceae were lower in the MPTP group. As shown in Fig. S9G, significant differences between the groups were also detected at the genus level. To present detailed information, we selected representative genera and used boxplots (Fig. S9H-K). Compared to the MPTP group, the relative abundance of Lactobacillus (p = 0.0399) (Fig. S9H) and Lachnospiraceae (p = 0.0427) (Fig S9I) was notably decreased in the C.R/MPTP group whereas the relative abundance of Citrobacter (p < 0.0001) (Fig. S9J) and Parabacteroides (p = 0.0015) (Fig. S9K) showed a significant increase following C.R plus MPTP treatment. These results collectively demonstrated a significantly altered microbiota profile in C.R/MPTP-treated mice.
To gain deeper insights into the role of the microbiota-gut-brain axis in the pathogenesis of PD associated with C.R infection, we conducted correlation analysis on a series of experimental data. Our findings revealed a significant inverse correlation between C.R colonization in the colon and DA level in the striatum (r = -0.6645, p = 0.0361) (Fig. 10E). A positive correlation was observed between fecal calprotectin expression and TLR4 protein expression in the striatum (r = 0.9387, p = 0.0017) (Fig. 10F). Furthermore, a significant negative correlation was identified between the ZO-1 integrity score and the number of TLR4+ cells in the SN (r = − 0.7947, p = 0.0327) (Fig. 10G). Additionally, a strong negative correlation was found between the relative abundance of Parabacteroides and the number of TH+ neurons in the SN (r = − 0.8272, p = 0.0217) (Fig. 10H). The study also revealed a negative correlation between the relative abundance of Lachnospiraceae and the number of Iba1+ cells in the striatum (r = − 0.8851, p = 0.0460) (Fig. 10I). Furthermore, a significant positive correlation was observed between the relative abundance of Lactobacillus and performance in the wire-hanging test (r = 0.9328, p = 0.0022) (Fig. 10J). Collectively, these correlation results provide strong evidence for the involvement of the microbiota-gut-brain axis in the development of PD, especially when C. R infection is present.
Effect of C.R infection or C.R plus MPTP administration on PD pathology. (A-B) The mRNA levels of inflammatory factors in the striatum were analyzed. n = 4. #p < 0.05, the MPTP group versus the control group; *p < 0.05, **p < 0.01, ***p < 0.001, the MPTP group versus the C.R/MPTP group. (C) Western blotting showing striatal levels of COX2. β-Actin served as the loading control. (D) Quantification of the relative expression levels of COX2. n = 4–5, *p < 0.05, **p < 0.01, ***p < 0.001. (E) C.R content in the colon were negatively correlated with the levels of the striatal DA. (F) The levels of calprotectin in the feces were positively correlated with TLR4 protein expression in the striatum detected by western blotting. (G) The ZO-1 expression in the colon was inversely correlated with TLR4+ cell numbers in the SN region. (H) The relative abundance of Parabacteroides was negatively correlated with TH+ neuron numbers in the SN region. (I) The relative abundance of Lachnospiraceae was inversely correlated with TH+ neuron numbers in the SN region. (J) The performances of the wire-hanging test were significantly positively the relative abundances of Lactobacillus













Add Comment