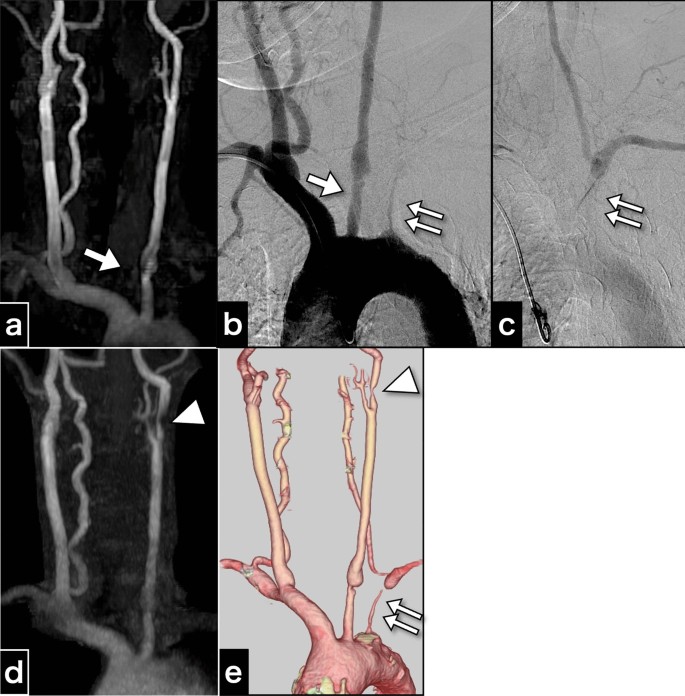
A 57-year-old right-handed female patient of East Asian descent, with poorly controlled atherosclerotic risk factors of dyslipidemia, diabetes mellitus, and severe hypertension, was prescribed atorvastatin calcium hydrate (5 mg once daily), and this medication regimen was maintained at the time of presentation. The patient had undergone health screening magnetic resonance angiography (MRA) (Fig. 1a). MRA revealed an asymptomatic pseudo-occlusion of the left subclavian artery and an irregular origin of the left common carotid artery (CCA). The aortogram showed left subclavian artery stenosis with subclavian steal phenomenon and irregularity in the origin of the left CCA (Fig. 1b, c). Cannulation to the CCA was avoided to minimize the risk of complications. The patient had no systemic inflammatory symptoms such as fever, arthralgia, or fatigue; vasculitis syndrome was not suspected. Because the patient was asymptomatic, no intervention was performed, and the patient was scheduled for routine follow up with medical treatment.
Cervical artery images obtained 3 years before carotid endarterectomy and just before carotid endarterectomy. a Magnetic resonance aortogram findings 3 years before carotid endarterectomy. b Aortogram findings 3 years before carotid endarterectomy. c The late-phase aortogram showing retrograde filling of the left vertebral artery and antegrade filling of the distal left subclavian artery. d Magnetic resonance aortogram findings just before carotid endarterectomy. e Computed tomography angiography findings just before carotid endarterectomy. The examinations performed 3 years before carotid endarterectomy a–c show an irregular origin of the left common carotid artery (arrow) and severe stenosis of the left subclavian artery (double arrow), and indications for the left subclavian steal phenomenon. The images just before carotid endarterectomy also depict progressive left internal carotid artery stenosis (arrowhead)
Three years later, MRA and computed tomography angiography (CTA) revealed new asymptomatic moderate stenosis of the left internal carotid artery (ICA) (Fig. 1d, e). Duplex ultrasonography revealed an accelerated peak systolic velocity of 217.1 cm/s. CEA was performed using a temporary intraluminal shunt.
Post-CEA clinical course
The patient regained consciousness from general anesthesia without any neurological deficits. However, on the first postoperative day, the patient experienced intermittent left monocular vision loss. Further, she experienced somnolence, orthostatic hypotension, and vomiting resulting from the vagal reflex, triggered by the operated carotid sinus. Continuous catecholamine infusion was needed to maintain her systolic blood pressure against orthostatic hypotension and vagal reflex.
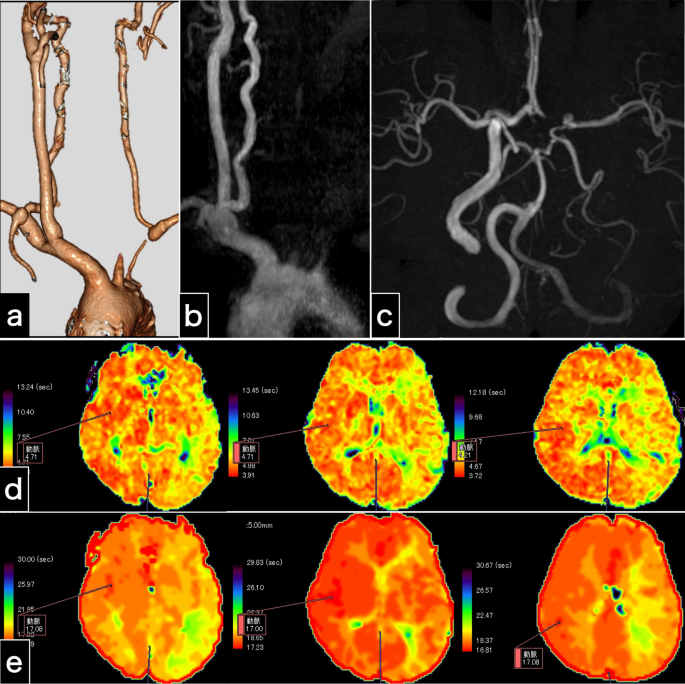
Radiological examination (Fig. 2) demonstrated left carotid artery occlusion between the origin of the CCA and the petrous ICA, making the site and cause of obstruction unclear. Cervical CTA (Fig. 2a) and MRA (Fig. 2b, c) revealed retrograde left vertebral arterial flow, indicative of cerebral blood flow diversion (stolen) to the left arm due to the subclavian steal phenomenon. Intracranial MRA revealed an entire intradural artery with collateral flow, a hypoplastic left anterior cerebral artery (A1), and distinct bilateral posterior communicating arteries (Fig. 2c), suggesting compensatory supplementary circulation via left posterior communicating artery from the right ICA and the right vertebral artery to counterbalance the cerebral blood flow insufficiency due to left carotid artery occlusion and left vertebral artery regurgitation. Diffusion-weighted imaging revealed only two high-intensity spots. Magnetic resonance imaging (MRI) perfusion revealed symmetrical cerebral blood flow and cerebral blood volume. However, MRI perfusion revealed a prolonged mean transit time (Fig. 2d) and time to peak (Fig. 2e). These findings indicated slight cerebral blood flow insufficiency. We diagnosed the patient with left carotid occlusion and orthostatic hypotension complicated by left amaurosis fugax. Accordingly, we explored treatment options to restore cerebral circulation.
Examinations 1 day after carotid endarterectomy. a Computed tomography angiography image shows left carotid artery occlusion and retrograde left vertebral artery flow. b The cervical magnetic resonance angiography image shows left carotid artery occlusion and no left vertebral artery antegrade flow. c Intracranial magnetic resonance angiography indicates no intracranial vessel occlusion and substantial collateral flow through the bilateral posterior communicating arteries. d, e Perfusion magnetic resonance imaging findings. The maps of the mean transit time (d) and time to peak (e) illustrate prolonged times in the left cerebral hemisphere
Treatment options to restore cerebral circulation
Vascular dissection was considered as the primary mechanism of occlusion. However, we could not visualize the external carotid artery, ICA, or CCA on radiological examination (Fig. 2); therefore, the dissection site could not be identified. The tip of the inserted internal shunt, distal edge of the endarterectomy, and vascular clamping site in the CCA were considered possible injured sites. Moreover, the onset of symptoms after an overnight period indicated possible secondary thrombus formation. The thrombus at carotid bifurcation and the CCA appeared to be extensive. Primary dissection at one of the possible sites and secondary massive thrombosis caused the occlusion. Thrombectomy via re-explosion wounds may not address dissection or thrombus outside the operating field. Endovascular treatment to non-contrast-enhanced carotid arteries could exacerbate dissection or cause distal migration of massive thrombus. Moreover, there were concerns that the narrowed origin of the left CCA might cause severe complications (Fig. 1e). Although catheterization of the CCA stenosis was considered, it could potentially compromise dissection of the aortic arch and the CCA origin in patients with chronic inflammatory diseases. However, 2 weeks later, pathological diagnosis of the resected intima confirmed atherosclerosis without chronic granulomatous changes suggestive of conditions such as Takayasu arteritis and giant cell arteritis.
Because the left vertebral artery was clearly visualized on cervical CTA (Fig. 2a) and the circle of Willis was well-developed (Fig. 2c), alleviating the subclavian steal phenomenon would restore whole-brain circulation (Fig. 1b, c). We determined that typical subclavian artery stenting, known to have a high procedural success rate [7, 8], is safer than direct carotid endovascular intervention, which is not well-visualized and has multiple underlying pathologies. This finding prompted us to perform left subclavian artery stenting as a safe palliative procedure. However, if the treatment had been ineffective, we would have performed left carotid intervention.
Endovascular treatment
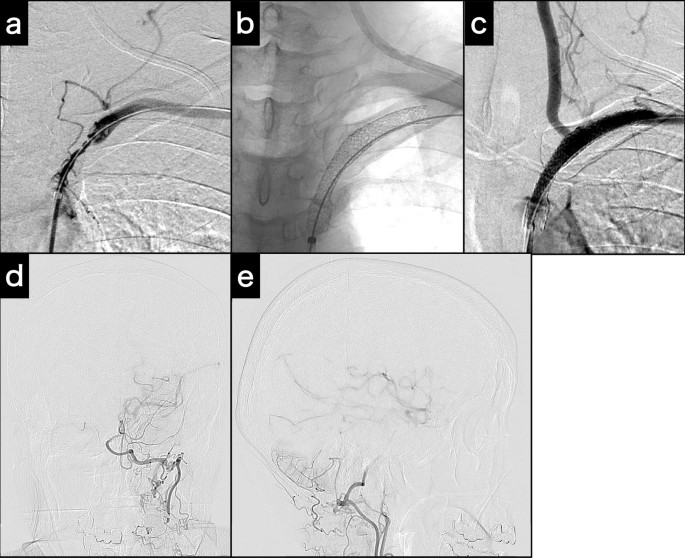
On the first postoperative day, endovascular treatment was performed under local anesthesia, with 200 mg aspirin and 300 mg clopidogrel as the loading doses. A 4-Fr 17-cm sheath was placed in the left radial artery, and a 4-Fr catheter was directed to the left subclavian artery to verify the condition of the left subclavian artery, followed by systemic heparinization. Initial angiography revealed a subclavian artery diameter of 7 mm. We planned to deploy a balloon-expandable stent, a 135-cm-shaft Express LD stent (70 × 37 mm; Boston Scientific, Marlborough, MA, USA) with a 6-Fr 90-cm guiding sheath via the right femoral artery.
First, the catheter tip of a 6-Fr, 125-cm inner catheter in the guiding sheath was applied to the subclavian artery ostium. A 0.035-in., 400-cm guidewire was introduced across the stenotic lesion. Then, the guidewire was inserted into the tip of the 4-Fr catheter from the 6-Fr catheter. Finally, the 4-Fr catheter was removed from the radial sheath, and the guidewire was passed through the sheath. Thus, the pull-through technique [8] was completed from the right femoral 6-Fr guiding sheath to the left radial 4-Fr sheath (Fig. 3a). A Mustang PTA balloon catheter (3 × 40 mm; Boston Scientific) was introduced through the guiding sheath and inflated. Because of a distal vascular dissection, two Express LD stents were then applied to the subclavian artery to cover the stenosis and dissection (Fig. 3b). At the end of the procedure, left subclavian artery angiogram revealed restoration of antegrade blood flow in the vertebral artery (Fig. 3c) and left middle cerebral artery blood flow via the left posterior communicating artery (Fig. 3d, e). The angiogram showed compensation for the reduced left ICA blood flow from the left subclavian artery.
Intraoperative examinations for subclavian stenting. a Angiogram acquired at the completion of the pull-through technique. b Fluoroscopy of the deployed and expanded stents. c Post-stenting angiogram demonstrates potent antegrade left vertebral artery flow. d, e Post-stenting left subclavian artery angiogram (d: Town’s view; e: lateral view) demonstrates left internal carotid artery and middle cerebral artery via posterior communicating artery collateral flow
Post-stenting clinical course
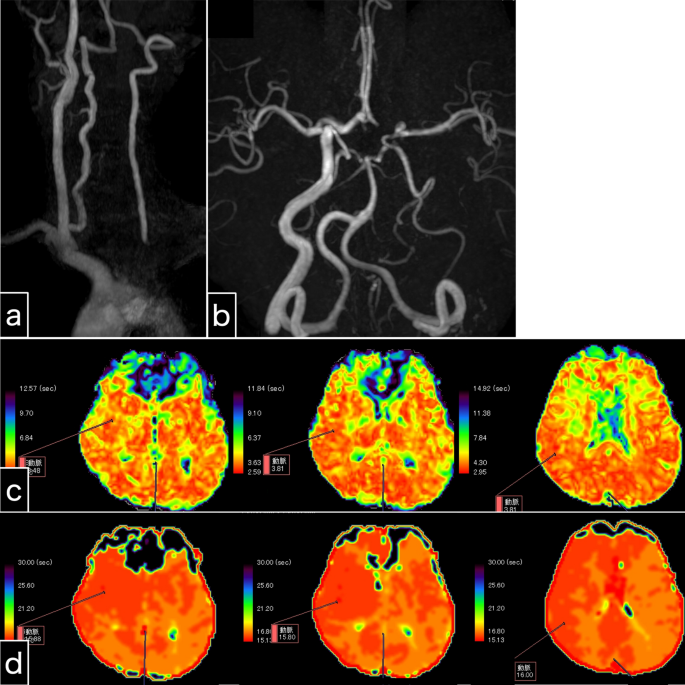
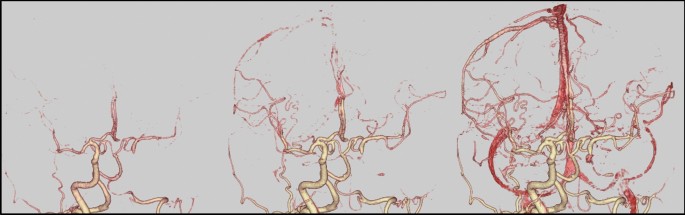
The amaurosis fugax resolved immediately after the stenting procedure. Somnolence and nausea resolved one day following endovascular treatment. The symptoms disappeared immediately after the present intervention, indicating their likely association with cerebral ischemia. However, orthostatic hypotension persisted, and continuous catecholamine administration was terminated 3 days following endovascular treatment. As a direct result of left subclavian artery stenting, left antegrade vertebral artery flow was clearly visible on MRA (Fig. 4a, b). As an indirect consequence, laterality appeared to be reduced on perfusion MRI when comparing the previous MRI (Fig. 2c, d) to the post-procedure MRI (Fig. 4c, d). Intracranial MRA (Fig. 4b) and four-dimensional CTA (Fig. 5) revealed collateral circulation from the left posterior communicating artery to the left cerebral hemisphere, with no collateral circulation via the left anterior communicating artery (A1). No new cerebral infarctions were observed on diffusion-weighted imaging.
Examination 1 day after left subclavian artery stenting. a Cervical magnetic resonance angiography shows potent antegrade left vertebral artery flow. b Intracranial magnetic resonance angiography shows that the left vertebral artery intensity is stronger than that of the pre-stenting magnetic resonance angiography. The collateral blood flow via the left posterior communicating artery, and not the anterior communicating artery, is depicted. c, d Perfusion magnetic resonance imaging. Maps of the mean transit time (c) and time to peak (d) reveal slightly prolonged times in the left cerebral hemisphere. Perfusion magnetic resonance imaging seems to show reduced laterality
The patient was discharged 2 weeks later without in-stent thrombosis. Dual antiplatelet therapy was continued for 3 months, after which aspirin alone was prescribed. For the past eight years, the patient has been attending our hospital for regular check-ups and has received prescriptions for atherosclerotic risk factors without any stroke episodes or any deficits. The patient was scheduled for routine follow-up with CTA and MRA every three months for the first year and every year thereafter. Although only a 30% width and shortest length in-stent re-stenosis have been observed, antegrade blood flow in the left vertebral artery remains maintained.





Add Comment