Biomass feedstock sourcing and preprocessing
Corn stover, poplar, and switchgrass were provided by Idaho National Laboratory (INL). Corn stover was harvest on October 23, 2018, in Iowa. Corn stover was received by INL on December 13, 2018, and stored in bale stacks 6 high and 3 wide. Corn stover was sampled for milling and processing on June 2, 2020. Corn stover was processed through a Vermeer BG480 tub grinder through 2-in. screen. 7600 lb of processed corn stover was shipped to NREL across 29 supersacks. Poplar was harvest on October 20, 2020, in Oregon. Poplar was received by INL on October 22, 2020. Poplar was sampled for milling and preprocessing on January 19, 2022. Poplar was processed through a Forest Concepts Crumbler M24 and sieved through a Forest Concepts Orbital Screen 2448-3 (1/4-in. screen on top and 3/32-in. screen on bottom). 6000lb of processed poplar was shipped to NREL across 15 supersacks. Switchgrass was a mixture of two different harvests at different locations. The first location was from Virginia and harvested on September 1, 2018 and received by INL on July 25, 2019. The second location was from Nebraska with an unknown harvest date and received by INL on March 3, 2020. Switchgrass was stored in bale mixture in bale stacks 6 high and 3 wide. Switchgrass was sampled for milling and processing on July 19, 2022. Switchgrass was processed through a Vermeer BG480 tub grinder through 3/4-in. screen. 9500 lb of processed switchgrass was shipped to NREL across 19 supersacks.
Biomass feedstock compositional analysis
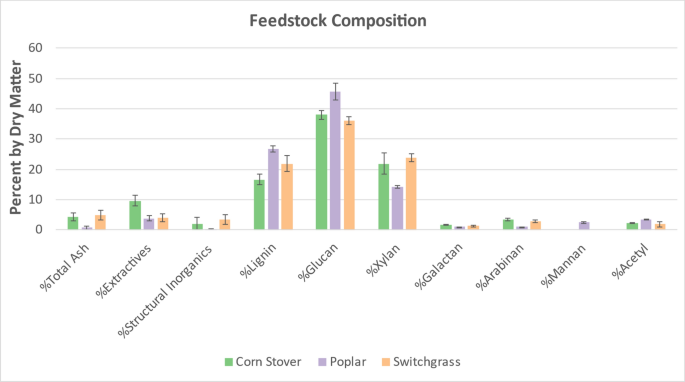
Three-six random supersacks of each feedstock were selected and randomly sampled at six different locations varying in depth, width, and height. Compositional analysis was performed using NREL’s Laboratory Analytical Procedure (LAP), “Determination of Structural Carbohydrates and Lignin in Biomass” [17]. Because the pretreatments were performed at pilot scale for which multiple supersacks were utilized, the compositions for each feedstock were averaged. The average compositions with their 95% confidence intervals are illustrated in Fig. 23.
Deacetylation
The DDR pretreatment processes used in this project consisted of three steps: deacetylation, solid–liquid separation via screw press, and disc-refining. The deacetylation was completed by soaking approximately 5 kg (dry basis) of biomass feedstock in a solution of sodium hydroxide of varying concentrations inside a custom built jacketed 90-L paddle reactor at 92 °C. The sodium hydroxide concentration used for deacetylation ranged from 60 to 100 g of sodium hydroxide per kg of dry biomass. Water was added to bring the total mass of the slurry to 45 kg. The residence time within the paddle reactor ranged from 120 to 240 min. The same sodium hydroxide concentrations and residence times were used for all three feedstocks for direct comparison. The resulting slurry of biomass and sodium hydroxide solution was then removed and fed through a screw press (VincentCorp, Tampa, FL). The deacetylated slurry was fed into the screw press via continuous feed through the top grate. Inside the screw press an air-pressurized sliding piston pushes the slurry through a dewatering press. The outside cone of the press has a screen which allows the lignin-rich deacetylated liquor, black liquor, to escape and be collected. The pressed deacetylated solids fraction collects and drops out of the bottom of the press into a collection container. The solids fraction collected ranged from 30 to 45% solids. A homogenized sample of each solids fractions were collected and submitted for compositional analysis, described in “Analytical” section. The black liquor samples were also collected and analyzed to quantify how much lignin, acetate, and carbohydrates from cellulose and hemicellulose were removed during deacetylation, described in “Analytical” section.
Disc-refining
The entire solids fraction collected was disc-refined with a 12-in. two-plate disc-refiner (Sprout-Waldron Operation, Koppers Company, Inc., Muncy, PA). The two refining plates are first secured. Once secured the motor is turned on which spins one disc while the second disc remains stationary. The gap space of the discs is adjusted to a gap less than 0.00 1in, the discs’ closest position. From there, the discs’ are adjusted to the desired gap space. The gap space for corn stover and switchgrass ranged from 0.005–0.015 in. and 0.001–0.005 in. for poplar. Poplar needed a smaller gap space between discs because the deacetylated poplar fell through the disc-refiner at 0.015 in. without sheering. Pressurized water was applied to the surface of the discs to help remove material. The pressed deacetylated solids were hand fed through the top grate of the feed hopper compression screw which conveys the material to the grinding discs. The material was sheered by the grinding discs and was dropped below the refiner to the collection vessel. The disc-refined material is then put through the screw press to dewater the collected material, as described in “Deacetylation” section. Disc-refining was used to help breakdown the cellulose and hemicellulose by sheering and refining the material into smaller particle sizes for better digestion during enzymatic hydrolysis [7,8,9]. The gap space between discs were varied to determine the impact on enzymatic hydrolysis conversion yields. A full table of DDR pretreatments is displayed in Table 1. A homogenized sample of the disc-refined solids was collected and submitted for compositional analysis as described in “Analytical” section.
Enzymatic hydrolysis
Enzymatic hydrolysis was performed on subsamples of each DDR solids fraction. Measuring the digestibility of the deacetylated disc-refined material was necessary to understand the overall impact sodium hydroxide concentrations, residence times, and gap space during disc-refining have on each feedstock during enzymatic hydrolysis. Enzymatic hydrolysis was performed in a laboratory scale roller bottle apparatus. This enzymatic hydrolysis procedure was similar to NREL’s LAP, “Low Solids Enzymatic Saccharification of Lignocellulosic Biomass” [18]. Changes were made to the method to allow for fermentation at a later date and to improve the overall economics. The enzymatic hydrolysis was done at 20% solids loading opposed to the 1% solids loading in the referenced procedure. Sodium azide and citric acid buffer were omitted from the enzymatic hydrolysis to allow for fermentations downstream. The pH was adjusted to 5.2 prior to the addition of the cellulase and hemicellulase enzyme cocktail. The pH was not controlled or monitored once the enzyme cocktail was introduced.
Novozymes Cellic® CTec3 (CTec3) and Novozymes Cellic® HTec3 (HTec3) were the cellulase and hemicellulase used during enzymatic hydrolysis. The total enzyme loading for enzymatic hydrolysis was 15 mg protein/g glucan. A blend of 80:20 (CTec3 vol: HTec3 vol) was used for all the enzymatic hydrolyses. CTec3 is an enzyme cocktail designed to break down cellulose to produce monomeric glucose while HTec3 is designed to breakdown hemicellulose to produce monomeric xylose.
1 mL samples were removed for each roller bottle on days 1, 2, 3, and 5 then submitted for HPLC analysis, described in “Analytical” section. The completed enzymatic hydrolysis slurries were submitted analyses described in “Analytical” section. Day 0 concentrations and percent solids were calculated from the pretreated slurry analytical data.
Fermentation
The fermentations were conducted on the same 36 hydrolysates generated by enzymatic hydrolysis after solid–liquid separation to remove remaining insoluble solids. Fermentations were conducted in 125-mL unbaffled shake flasks. All fermentations were conducted in duplicate when sufficient hydrolysate was available. The filtered hydrolysates from the different samples contained a wide range of compound concentrations.
Due to the large number of hydrolysates and the desire to perform the fermentation tests in duplicate, it was not possible to logistically conduct all the shake-flask fermentations in a single campaign. Therefore, four separate campaigns were conducted. In each campaign, each hydrolysate was run in single flasks.
A single inoculum flask was prepared for each campaign, which was subdivided and used to inoculate all flasks in each fermentation campaign once the cell growth in the inoculum flask achieved an optical density (OD) of > 5.0. In general, 32 g of filtered hydrolysate and 8 g of combined inoculum and media (rich media [RM] containing 20 g/L glucose, 10 g/L yeast extract, and 2 g/L KH2PO4) were combined to achieve a target starting OD of 0.5 in the fermentation flasks. The starting pH prior to inoculation was 6.0 (hydrolysate adjusted with ammonium hydroxide) and was not controlled or adjusted during the shake-flask fermentations. Once inoculated, each flask was sampled at 0, 6, 12, 24, 48, and 72 h. A typical arrangement for the shake flasks for a single campaign inside of a temperature-controlled orbital shaker (temperate was controlled at 30 °C).
Time-point samples taken during the fermentation were centrifuged and supernatants filtered through a 0.2-μm syringe filter before being placed in high-pressure liquid chromatography (HPLC) vials. The samples were immediately centrifuged, and supernatants filtered through a 0.2 μm syringe and submitted for HPLC analysis as described in “Analytical” section.
The YC-1 strain of Z. Mobilis in dictated by the amount dissolved oxygen during agitation of the fermentation [14]. Both acetoin and 2,3-BDO have the same theoretical production yields and both can be upgraded to fuel intermediates. Therefore, the production of acetoin and 2,3-BDO were summed as overall product.
Analytical
The total solids analysis of the deacetylated feedstock biomass, black liquors, enzymatic hydrolysis slurries and liquors, and fermentation liquors were performed using NREL’s LAP, “Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples” [19]. Due to the solids and black liquor being caustic, the weighing procedure was changed to using a plastic pan to prevent the sodium hydroxide from reacting with a metal pan. The black liquor samples were also dried in a 40 °C vacuum oven for three days instead of 12 h in a 105 °C oven. Total solids of whole slurries and liquid fractions were used to calculate insoluble solids fractions which were used to calculate procedural yields.
The chemical composition analysis of the black liquor, enzymatic hydrolysis liquors, and fermentation liquors followed NREL’s (LAP), “Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples” [20]. All standard high-performance liquid chromatography (HPLC) methods were ran on Agilent 1100/1200/1260 Infinity II/1290 Infinity II system. Due to the high lignin and sodium concentrations of black liquor, some of the procedure was modified. Instead of using a HPLC for quantification of sugars, a Dionex ICS-5000+ converted for high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD). The eluent was 0.001 M KOH, 50 µl injection, a flow rate of 1.5 mL/min, a Dionex SA-10 column at 45 °C, and a detector temperature of 35 °C. The organic acids and byproducts were quantified using the method in the NREL LAP, but due to the acidic eluent, the black liquor was acidified to 4% sulfuric acid and filtered to remove the precipitated lignin. The method was also modified to quantify 2,3-BDO and acetoin for quantification of fermentation products.
The chemical composition of the solids after deacetylation, disc-refining, and enzymatic hydrolysis were performed using NREL’s LAP, “Determination of Structural Carbohydrates and Lignin in Biomass” [17]. Because the solids fraction had residual sodium hydroxide and black liquor byproducts present, the solids fraction was washed with water to a neutral pH and dried prior to analysis. Similarly, enzymatic hydrolysis solids had residual sugar present and were washed in order to quantify the remaining compositional solids fraction. All HPLC methods were ran on Agilent 1100/1200/1260 Infinity II/1290 Infinity II system.
Techno-economical analysis
Using a process simulation model based in Aspen Plus V10 with the functionality to reflect deacetylation and mechanical refining or disc-refining, a techno-economic analysis was conducted leveraging NREL’s published biochemical sugar model [21] with modifications for reflect experimental data presented herein and updated to more recent cost and financial parameters [13]. The TEA modeling was performed on all three feedstocks, corn stover, poplar, and switchgrass for all 12 variable conditions, sodium hydroxide concentrations, residence time during deacetylation, and gap space for disc-refining. Due to the fermentations being run in a shake flask setup, which is not representative of batch fermentation kinetics or yields at commercially relevant conditions (as intended to be reflected in the TEA model), the scope of the TEA analysis was limited to sugar production, excluding fermentation or other downstream conversion steps to intermediate/finished fuels. However, trends in resulting minimum sugar selling prices (MSSPs, the selling price for clarified sugars following solid–liquid separation as required to achieve a 10% rate of return at zero net present value) can be extrapolated to comparative economics that would be expected for downstream fuel production. All methods and assumptions for TEA modeling are consistent with previously published practices [13].





Add Comment