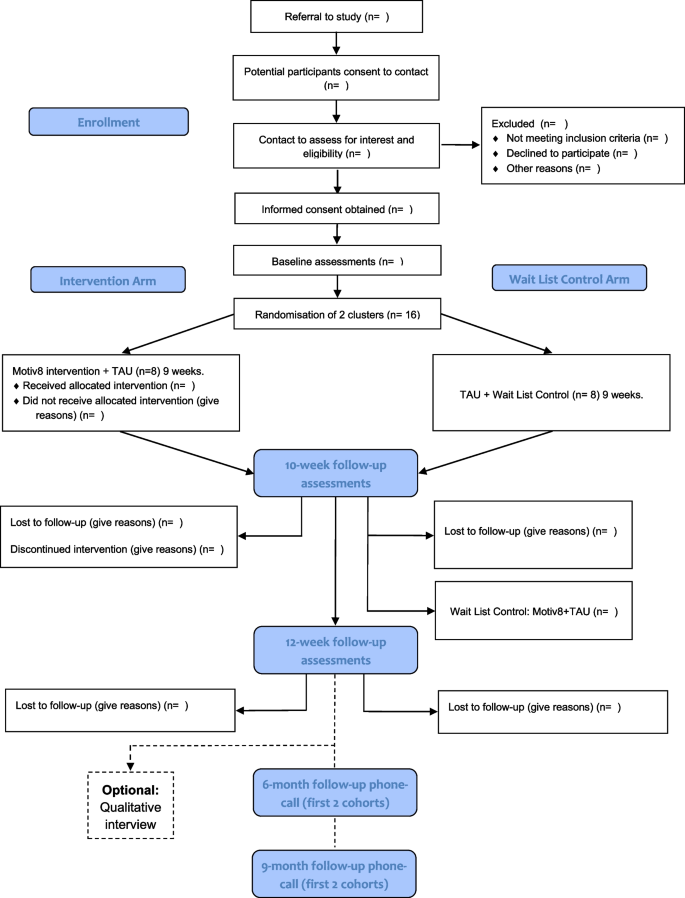
Trial design and flow chart
All procedures and conduct of this trial will be conducted in line with the CONSORT Extension to Randomised Controlled Trials [31]. The design is a prospective, single-blind, cluster-randomised controlled feasibility trial with two conditions; weight management intervention (Motiv8) plus treatment as usual (TAU), versus TAU waitlist control (with Motiv8 delivered after TAU). The study will take place in adult secure, forensic NHS mental health services. TAU will be measured throughout, and no treatment will be withheld from participants. Assessments will be completed at baseline (pre-intervention), 10 weeks (the week after participants finished Motiv8 or TAU), and again 12 weeks after the end of the intervention period (12-week follow-up). A nested qualitative study will explore the subjective experiences of taking part in Motiv8 and the acceptability of the intervention. See Fig. 1 for a summary of the trial design. The trial is prospectively registered on the ISRCTN registry: ISRCTN13539285 (ISRCTN-ISRCTN13539285: Motiv8: A weight management intervention for adults in secure mental health inpatient services). An independent Trial Steering Committee (TSC) and Experts by Experience Group have been established to provide ongoing guidance and oversight of the study.
Participants
Participants will be current service users of low or medium-secure, adult forensic inpatient services in Greater Manchester Mental Health NHS Foundation Trust (GMMH NHS FT). The service provides individualised care and treatment for people with severe and enduring mental health disorders. We will aim to recruit a total of 32 participants forming four cohorts of Motiv8. Sample size is based on pragmatic limitations associated with the need to keep groups small due to the complex needs of service users, and the time constraints of funding. Following on from successful pilot work, clinical teams will be approached to identify eligible individuals to refer to the research team. The study will be advertised widely across the trust to service users and staff (such as through internal bulletins, social media, and recruitment materials). Clinicians will discuss the study with their service users and provide consent to contact. Researchers will provide potential participants with enough information to permit them to provide informed consent before taking part.
The inclusion and exclusion criteria are as follows:
Inclusion
-
Adult inpatient (at least 18 years old) at mental health medium or low secure unit at GMMH NHS FT.
-
Mental health diagnosis requiring treatment from secure services.
-
Capacity to provide informed consent.
Exclusion
-
Inability to provide informed consent in line with ethical requirements.
-
Previous Motiv8 participant from the pilot work.
-
Insufficient command of English/communication difficulties preventing engagement in written informed consent, the validity of research assessments or understanding of the programme.
Wards must have 8 people identified (maximum amount for each Motiv8 group) before randomisation. Each cohort will aim to contain people from the same ward to avoid conflict between patients and avoid contamination of the control groups; this decision is based on previous work in secure units, restrictions on movement and the internal pilot [12, 15, 17]. Individuals from the pilot phase and PPI consultations claimed being from the same unit is beneficial as it reduces anxiety being with people they know and avoids conflict between wards. Ongoing restrictions due to the COVID-19 pandemic also prevent mixing across different wards. In the instance that fewer than 8 people from the same ward are interested, or someone moves ward during the intervention period two wards may be combined to make up the cohort using a contingency plan developed within the study team. In line with principles of informed consent all participants will be made aware of their right to withdraw from the study at any point should they change their mind.
Randomisation and blinding
Individuals will be cluster randomised by cohort, using minimisation to ensure balanced distribution. Following written consent, cohorts will be randomised using a free web-based system (www.sealedenvelope.com). Allocation will be communicated to the CI, study management team and facilitators but not research assistants, statistician or health economist. Participants will be informed of their randomisation status by letter or via clinicians, communicated via the administrator.
Blinding of allocation will be maintained for research assistants until all outcome measures for all subjects have been collected. Blindness will be maintained using a range of measures (e.g. separate offices for facilitators and researchers, protocols for answering phones, secretarial support). Unblinding will be communicated to the CI, and if possible, future assessments will be conducted by a blinded assessor. This may not always be possible due to the trial being cluster randomised, and therefore unblinding will occur on a cohort basis. Maintaining rater blindness to treatment allocation is crucial, and the TSC will be consulted in the instance that any unblinding occurs and implement corrective action if necessary.
Assessments
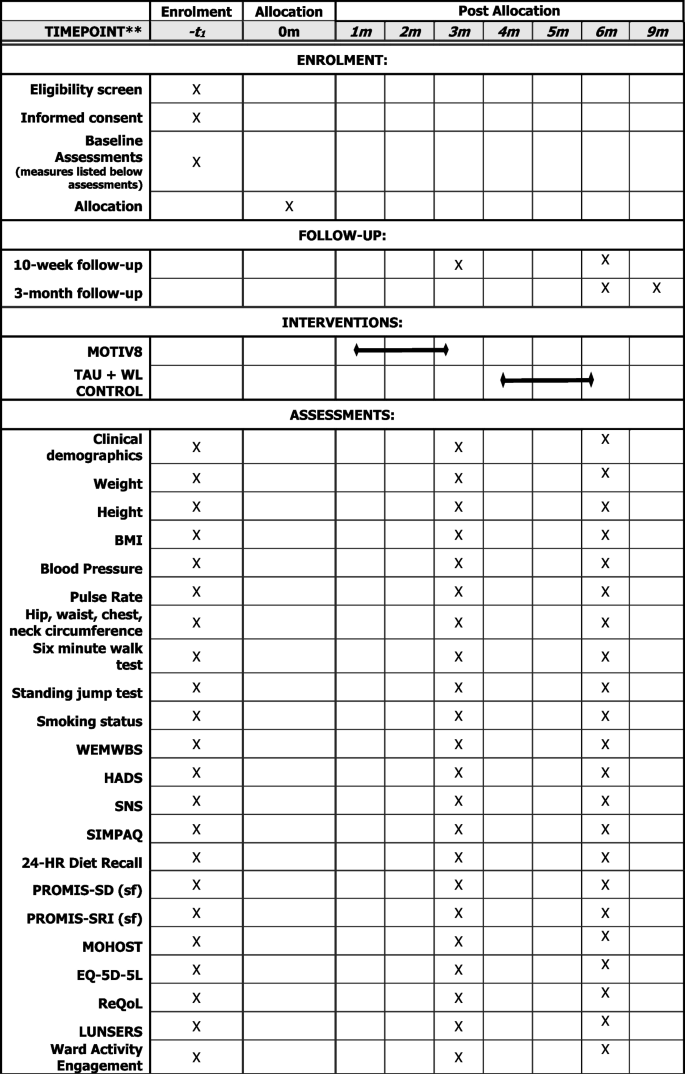
Assessments will be conducted at three timepoints; baseline (pre-intervention/waitlist), week after intervention/waitlist (10 weeks) and follow-up (12 weeks after the end of the intervention). Basic sociodemographic and clinical information will be collected at baseline (e.g. age, gender, ethnicity, diagnoses, physical health conditions, duration of illness, length of admission). Clinically relevant information will also be collected at all three timepoints (e.g. medication, service use, treatment plan), (See Fig. 2).
The following measures will be collected:
-
Physical health assessments
BMI (Height/Weight), Blood Pressure, Pulse Rate, Hip/Waist/Chest/Neck Circumference, Cardiorespiratory fitness (using a vo2 sub max proxy test 6-min walk and standing jump test).
-
Mental health assessments
Warwick Edinburgh Mental Wellbeing Scale (WEMWBS; [32]), Depression and Anxiety (Hospital Anxiety and Depression Scale; [33]), Negative Symptoms (SNS; [34]).
-
Behavioural assessments
Physical Activity (SIMPAQ; [35]), Model of Human Occupation Screening Tool for nutrition sessions (MOHOST; [36]), 24-h diet recall [37], Sleep (PROMIS SD Short Form 8-item; PROMIS SRI Short form 4-item; [38,39,40].
-
Measures to support future economic evaluation
Health status (EQ-5D-5L; [41]), Quality of Life (ReQoL; [42]), the Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS; [43]), Engagement with ward activities and care.
Assessments are a mixture of self-report and researcher administered and may be collected over several sessions, with an aim of completing all within a week prior to or following the intervention. A nested qualitative study will be conducted with a subsample of participants (n = 10) after their final assessment to explore their experiences of taking part. Interview schedules will be developed with service user and carer input. To establish proof of concept of a longer follow-up period for a definitive study the first two cohorts will aim to be contacted after 6 and 9 months following the end of the intervention. Participants will be asked if it was a definitive trial whether they would be willing to complete assessments again.
The Motiv8 intervention
Motiv8 is a 9-week, intensive programme co-developed with service users to improve the cardiovascular health of patients in secure inpatient units. The intervention aims to increase activity levels, improve diet, and use psychological guidance and techniques to maintain good physical health using goal-based techniques. Motiv8 is a multidisciplinary intervention encompassing several components to support physical health including exercise sessions, cooking/nutrition classes, physical health education, psychology sessions, sleep hygiene support, and a medication review (see Table 2 for an example schedule). Motiv8 will be facilitated and delivered by occupational therapists, dietitians, psychologists, pharmacists, physicians, exercise/sport professionals, nurses, and support workers. Trained service users assist with programme delivery to provide peer support and promote participant morale. An intervention booklet is provided consisting of resources, activities and prompts for goal setting/review of progress. A particular emphasis is placed on achievements and community, and participants will attend an awards ceremony upon completion.
The content of the sessions and all materials are agreed upon by the MDT team and research team. Exercise sessions are supervised and guided physical activity sessions lasting up to 1 h which include a mixture of cardio and strength-based exercises completed in onsite gym facilities and outdoor areas which gradually increase in difficulty. The cooking and diet sessions lasting 90 min are guided by group preferences and dietary requirements (e.g. halal, vegetarian). Recipes are selected by the group who are guided through the cooking process, having discussions about portion sizes and healthy food swaps. The group then engaged in a shared dining experience and sampled the food they cooked. Physical health education/sleep sessions are 1 h and interactive with a focus on providing education on looking after physical health, side effects of medication and ensuring positive sleep habits. Psychology sessions are interactive group sessions that are based on psychological theories of motivation and behaviour change, including problem-solving and goal setting.
Findings from successful pilot work across five cohorts indicated that Motiv8 may be feasible and beneficial to service users on secure units (unpublished). The purpose of this trial is to gather more information regarding the feasibility and potential efficacy of Motiv8 as a weight management intervention.
Waitlist control group
Participants randomised to the waitlist control arm will receive their usual treatment and assessments at the same time points as those receiving Motiv8. Following the 10-week follow-up participants will receive Motiv8. Typical treatments for this group include pharmacological treatment, psychological therapy (group/individual), and various occupational therapy activities. They will be able to access gym facilities and be encouraged to eat well in line with usual care, and clinicians will be informed not to withhold any treatments whilst they await Motiv8. Any treatments received during the study will be recorded. After completing pre-/post-assessments individuals will receive Motiv8. This is an enhancement to routine care.
Staff evaluation
Staff across the secure services will be asked to complete questionnaires to assess their attitudes and beliefs about physical health, and the ward atmosphere (ESSEN-Climate Evaluation Schema (ESSEN-CES; [44]), Metabolic-Barriers, Attitudes, Confidence and Knowledge Questionnaire (M-Back Questionnaire; [45]). Staff on non-participating wards will also be encouraged to complete questionnaires to allow comparison of the overall ward environment when not taking part in a physical health trial. Clinical staff will be encouraged to provide feedback in a specially designed questionnaire, or interviews after Motiv8 has taken place.
Fidelity and facilitator evaluation
Fidelity assessments will be undertaken to see whether the intervention can be delivered according to protocol and inform a definitive trial. Adherence checklists specific to each component will be completed at the end of each session by facilitators. Assessment of facilitator performance will be conducted throughout the trial, during regular monitoring and supervision. Feedback will be collected from facilitators at the end of the trial and feedback strategy sessions will be held with facilitators and the research team. Intervention fidelity assessments, feedback and outcome measures will be used to inform a definitive trial.
Analysis
A detailed Statistical Analysis Plan will be drafted by the study statistician prior to data analysis and a Health Economics Analysis Plan will be drafted by the study health economist. Quantitative analysis will be conducted according to intention-to-treat and reported according to CONSORT guidelines for cluster randomised pilot and feasibility studies [46], including the numbers of prospective participants who were approached, deemed eligible and consented. The number of participants who received their intended treatment (including which elements of it) will be reported.
There are no formal stopping rules for this study as the primary aim is to assess feasibility. The primary focus will be on tabulated and graphical summaries of key indicators of success of the study, e.g. recruitment, engagement, retention and satisfaction with the Motiv8 intervention (participant and facilitator). Where applicable these will be reported with a 95% confidence interval. All adverse events will be reported. We will summarise the baseline demographic and clinical characteristics of each cohort by trial arm. The completion rate of assessments will be reported, as well as descriptive characteristics such as mean (SD), median (IQR) or number (percentage).
To determine the potential utility of the Motiv8 intervention, an appropriate regression model will be fit to the data, using our intended primary outcome (weight), with a trial arm as a covariate controlling for gender and ward type. P-values will not be reported as this study is not designed to test effectiveness—instead, 70, 80 and 90% confidence intervals for the difference in weight between Motiv8 and TAU. Alternative proposed primary outcomes such as cardiovascular fitness, physical activity and well-being will be explored.
As Motiv8 is delivered in cohorts, a degree of intra-cohort correlation will exist in the outcomes. A sample size calculation for a definitive trial will require an estimate of the intra-cohort correlation. The correlation in this study will be investigated, but the number of cohorts is likely to be too small to obtain an accurate estimate. We will use descriptive statistics to inform the design of the economic components of the definitive trial, based on completion rate and summaries of relevant data (ReQOL, EQ-5D, service use and engagement, treatments).
Qualitative interviews will be audio-recorded and transcribed verbatim. Thematic analysis will be conducted according to the five-phase procedure described by Braun and Clarke [47]: familiarisation; initial code generation; searching and identifying themes; reviewing themes; and defining and naming themes.
Patient and public involvement
Extensive PPI work underpins this study. Motiv8 has been co-developed and co-produced with service users and staff, from conception and ongoing iterative updates have been used to incorporate feedback from previous cohorts. Multiple discussion groups and consultations have been held with service users in secure services to refine the protocol and inform the design pre- and post-funding allocation. All research materials, such as information sheets, branding materials and recruitment leaflets have been co-produced with service user input. The core research team consists of investigators with lived experience and parent/carer representatives. PPI representatives will ensure the research is appropriate and sensitive to the needs of service users. People with lived experience will assist with the delivery of the intervention and provide peer support. Following the award of the grant, an independent expert by-experience group has been set up consisting of people who have previously used secure services. This group meets bimonthly and will continue to advise on all aspects of study progress (e.g. recruitment, analysis, and dissemination).
Progression criteria
A red/amber/green criterion for progression to a full trial will be used, with a stop/refine/go approach. This will likely be recruitment > 80% (green), 60–79% (amber), < 60% (red) of planned target; adherence > 70% (green), 50–69% (amber), < 50% (red) attendance at planned sessions; retention within the study > 75% (green), 60–74% (amber), < 60% (red) completion of proposed primary outcome for the definitive trial (weight). Retention to the proposed primary outcome measure for a definitive trial (change in weight) will be monitored.



Add Comment