Surveillance, follow-up, screening, and enrollment
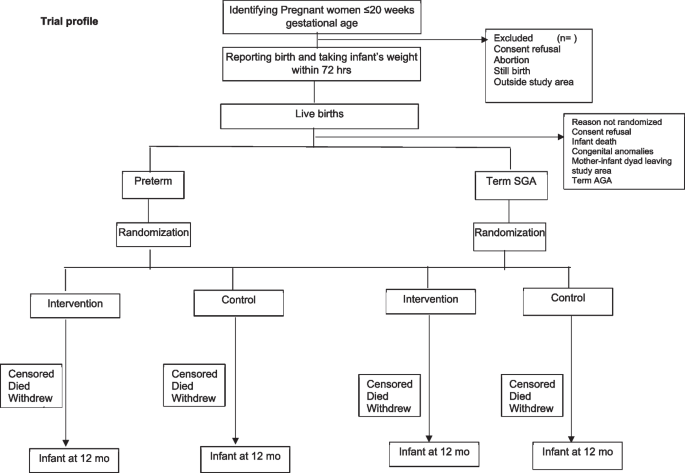
A pregnancy surveillance team (PST) conducts a door-to-door survey to list all pregnancies within 20 weeks of gestation based on the last menstrual period (LMP) or previous ultrasound and takes consent for ultrasound, regular contacts during pregnancy, and birth weight measurement within 72 h of childbirth. The team offers to facilitate the dating ultrasound in designated USG centers and transportation for the same. The details of pregnant women with gestational age < 20 weeks based on ultrasound are communicated to the pregnancy follow-up, screening, and enrolment team (PSE) (Fig. 1).
During follow-up, pregnant women are counselled by field assistants (FAs) to register and deliver in a hospital, attend regular antenatal care clinics, consume an adequate diet, recognize danger signs in pregnancy, and initiate of breastfeeding early after birth. The field assistants periodically contact the identified women over the phone (through home visits if phone calls are unsuccessful), more frequently in the last trimester. For all live births, the team measures the baby’s weight within 72 h of birth and screens using inclusion and exclusion criteria. If the inclusion criteria are met, there are no exclusion criteria, and consent is given; the mother-infant pair is randomized to the intervention or control arm in the relevant stratum (preterm or term SGA). Post enrollment, baseline information is collected, including sociodemographic characteristics and recent childbirth related details including care received after delivery.
Randomization, allocation concealment, and masking {16a, 16b, 16c, 17a}
The randomization list was prepared by an independent statistician using random permuted blocks of varying sizes, stratified by preterm and term SGA infants. The arm allocation is done using a web-based system at the time of enrolment. There are no additional criteria for discontinuation or modification of allocated intervention. Masking of the study teams is not possible because of the nature of the intervention. However, attempts are made to keep the independent outcome ascertainment team unaware of the arm allocation, to the extent possible.
Intervention delivery
The PSE team informs the intervention delivery team about the infants randomized to the intervention arm. The intervention delivery teams conduct the first visit within 24 h of enrolment.
The intervention delivery visits are designed to accommodate the implementation of various components of intervention such as health, nutrition, and psychosocial in a single visit. On a particular visit, the focus is either on nutrition and health of the infant and mother or early child stimulation and responsive care and psychosocial care and support of the mother, with adjustments made as necessary. This intervention includes counselling on early and exclusive breastfeeding, expressing breast milk, kangaroo mother care, responsive feeding, micronutrient supplementation, hand washing, family planning, and immunization. The schedule includes 11 visits (days 1, 3, 7, 10, 11, 14, 17, 21, 22, 24, and 28) in the first 28 days, followed by visits every 2 weeks in months 2 and 3, and monthly visits in months 4, 5, and 6. In addition to providing counselling and support, the team also acknowledges and praises good practices.
During each visit, FAs ask about the well-being of both the infant and the mother. If the infant needs urgent care, the FA informs the supervisor and facilitates referral to the nearest health facility based on the family’s preference. The FA checks the mother’s understanding of the counselling messages and summarizes the key messages at the end of each visit.
If any problem with breastfeeding or complementary feeding is identified, a lactation counsellor or nutritionist intervenes to resolve the problem. If the problem is not resolved, the infants are referred to the pediatrician at the collaborating hospital.
The intervention delivery team FAs are measuring compliance with intervention during their home visits. This is done by both asking questions to the mother and observing empty food supplement packets or counting remaining tablets.
The pediatrician at the study clinic, located within the collaborating hospital, provides care for the infants in the intervention group and assesses infants with growth failure and associated illnesses. The lactation counsellor and nutritionist conduct home visits to assess breastfeeding and complementary feeding practices and counsel the mothers. Mothers of all infants with growth failure are assessed by a psychologist for any psychosocial problems that may pose hurdles in taking optimal care of the infant.
Strategies to improve adherence to interventions {11c}
A system for electronic surveillance has been established to monitor infants who need extra care to ensure high adherence to intervention.
Relevant concomitant care permitted or prohibited during the trial {11d}
Both groups of participants (intervention and control) have the freedom to utilize the standard care pathways, which include complimentary services from the government’s health system.
Process evaluation and quality control
Two types of visits are conducted: observed visits and independent visits. Observed visits closely monitor worker activities, including family interactions, counselling quality, and procedure adherence. Coordinators perform monthly independent visits to ensure team adherence and accurate data collection. During pregnancy surveillance, activities involve observing rapport-building, survey-related messaging, assessing LMP, and consenting. During pregnancy follow-up, coordinators monitor the process of delivering counselling messages over the phone and handling inquiries from the participants. For screening and enrollment, criteria assessment, consenting, and anthropometric measurements are observed. Observation of outcome ascertainment focuses on the adequacy of anthropometric assessment methods. During intervention delivery, supervisors observe feeding sessions, breastfeeding practices, and child stimulation activities for the first 6 months, and complementary feeding practices from 7 to 12 months, with all details related to compliance documented at each visit.
Supplements are provided every 2 weeks, and at the time of delivery, the team asks questions on supplement consumption since the last visit and counts the remaining tablets to monitor compliance.
Outcome ascertainment {18a}
Infants in both the intervention and control arms are visited at home by an independent outcome ascertainment team in pairs, at infant ages 1, 3, 6, 9, and 12 months, to measure weight, length, and head and mid upper arm circumference (MUAC); assess infant care practices; and document the prevalence of reported illnesses in the previous 2 weeks and care-seeking for illness and hospitalizations since the last visit. Additionally, the team measures the weight and MUAC of mothers at 2, 6, and 12 months postpartum and assesses postpartum depression among mothers using the Edinburgh Postnatal Depression Scale (EPDS), which has been validated for use in India [63].
Weight measurements are obtained using digital weighing scales (Seca model 354; California, USA) with an accuracy of up to 10 g, while infant length measurements are taken using infantometers (Seca model 417; California, USA) with a precision of 0.1 centimeters. Head and MUAC are taken using a measuring tape (model 212; Seca, California, USA) [64,65,66].
Information on household consumption and expenditures (monthly expenses on food, rent, health care, utilities, maintenance, fuel, reimbursement of loans, and helpers; education and health care expenses in the preceding three months; annual expenses on insurance, and clothes) will be collected at enrollment and 6 and 12 months of age. Neurodevelopmental assessment is done by trained and standardized psychologists. Cognitive, motor, language, and socio-emotional development will be assessed at 12 months of age using the Bayley Scales of Infant and Toddler Development III (BSID-III) [67]. Infant temperament will be assessed at 12 months of age using the Infant Temperament Scale [67, 68]. The child’s home environment will be assessed using the Home Observation of the Environment (HOME) questionnaire (Infant and Toddler version [69]) by the trained field team through physical home visits. Additionally, the Global Scales for Early Development (GSED) scale will be used at 6 and 12 months to assess child development across multiple domains, including cognitive, motor, and social-emotional development. Blood samples (~10 ml) will be collected at 12 months of age for micronutrient assays. The samples will be centrifuged, and serum and blood pellet stored at – 80 °C in the field office. The micronutrient concentrations will be measured in accredited laboratories.
Dietary assessment is done using: a food frequency questionnaire (FFQ) and 24-h dietary recall (subsample) at 9-month and 12-month outcome visits. Mothers of infants will be asked to provide information regarding their child’s food consumption in the previous 24-h duration. The collected data from these recalls will be entered into the DietCal software [70].
Ultrasounds for assessment of preterm birth are done at designated ultrasound centers. A trans-abdominal USG is scheduled between 9 and 13 weeks of gestation to estimate gestational age calculated by fetal crown length. If CRL is > 95 mm, femur length and head circumference are used to assess gestational age. All digital images are taken by trained radiologists according to intergrowth standards. Ten percent of all USG scans are randomly selected and sent to external reviewer for quality assurance.
Participants who discontinue the intervention will be treated as censored data and will be included in the analysis up to the point of discontinuation.
Training and standardization
Staff are trained in the overall study objectives, strategies, and in their job responsibilities. Additionally, each team receives intensive training in their area of work along with training in Good Clinical Practice (GCP) guidelines.
Inter- and intra-observer standardization exercises for weight, length measurements, head circumference, and MUAC were conducted at the beginning of the study and will be repeated every 6 months. Weighing scales and infantometers are calibrated daily using standard weights and length measurement rods [56].
The psychologists undergo inter- and intra-observer standardization exercises for Bayley assessments. In addition, 10% assessments will be done by 2 psychologists. Agreement between the measurements assessed by the intra-cluster correlation coefficient (ICC) and by calculating Lin’s concordance correlation coefficient [71].
Study oversight
Coordinators designated for each activity oversee the work of their teams. Weekly status reports are shared with the investigators. Periodic review meetings are held between the study teams, coordinators, and investigators.
The Centre for Intervention Science in Maternal and Child Health (CISMAC) is responsible for the oversight of the study. Technical staff from CISMAC interact with the investigators through monthly conference calls and twice-yearly site visits to review the study progress.
Data management {#19}
A data management center is set up in the field office where real-time data is transferred to the server. Data is captured electronically on tablets and mobile phones and uploaded to an access-controlled cloud server. Range and logical checks have been incorporated to reduce errors. Additional logical and across-form checks are run twice weekly. Queries generated are given to study teams, and necessary corrections to the database are logged.
Data safety monitoring committee (DSMC) {21a, 21b}
CISMAC has established a DSMC to oversee the study’s progress and evaluate the safety of the intervention. The members include an epidemiologist, a statistician, a pediatrician, and a social scientist. The committee reviews data on adverse events to supplements and deaths quarterly and meets twice a year. The committee suggested that trial-stopping rules will be dependent on the number of interim analyses that may be planned. For efficacy, interim analysis each of the two strata O’Brien Fleming’s rule will be followed. This rule is a group sequential method that sets increasingly stringent boundaries for statistical significance as the data accrue, thus controlling the overall type I error rate. For safety interim analysis in each of the two strata, Pocock’s rule will be followed. This rule uses a constant boundary for statistical significance, allowing for regular monitoring of the data without inflating the type I error rate. The first interim analysis will be conducted when 50% of enrolments have occurred in both strata. These stopping rules will ensure that the trial can be stopped early if there is clear evidence of benefit or harm, while also maintaining the integrity of the study results. The approach taken to these analyses will be rigorous and will adhere to the highest standards of statistical practice.
Harms {#22}
This is a low-risk study, and serious adverse events are unlikely. All deaths of enrolled participants will be reported to the local ethics committee and the DSMC.





Add Comment