Introduction
Fluorescent probes have been regarded as an easy and powerful approach in biological fields such as cell imaging, biosensors, and early cancer diagnosis at the molecular level due to their rapid response, specificity, real-time capability, and high reactivity both in vitro and in vivo (Danylchuk et al., 2021). Generally, fluorescent probes are mainly composed of a fluorophore group and a recognition group, which are bridged by a linker. The intensity of emission wavelength could be tuned by the interaction between targets and reactive recognition groups, normally based on the mechanisms including fluorescence resonance energy transfer (FRET), intramolecular charge transfer (ICT), twisted intramolecular charge transfer (TICT), photo-induced electron transfer (PET), aggregation-induced emission (AIE), and through-bond energy transfer (TBET) (Fang et al., 2023). Typically, ICT is one of the traditional sensing mechanisms, and ICT-based fluorescent probes have been obtained and applied in organisms due to their biocompatibility, good stability, tunable spectrum, and easy processability (Jana et al., 2021). TICT fluorescent probes usually exhibit low fluorescence efficiency in solution but show significantly increased fluorescence emission intensity in specific environments such as inside organisms due to TICT. Therefore, TICT fluorescent probes have high sensitivity in detecting microenvironment changes and molecular conformational changes within biological systems. PET fluorescent probes are usually composed of a fluorophore and an electron acceptor, and their fluorescence can be switched on/off through the PET process. When the electron acceptor binds with the target molecule, the PET process is affected, leading to changes in fluorescence emission intensity. This property allows PET fluorescent probes for use in quantitative and positional analyses of biomolecules. AIE fluorescent probes emit weak or no fluorescence in dilute solutions but exhibit strong fluorescence in high concentrations or aggregated states. This aggregation-induced emission phenomenon makes AIE fluorescent probes advantageous in applications such as biological imaging and biosensors, with features including high sensitivity, low background signal, and excellent optical properties. TBET fluorescent probes modulate fluorescence through energy transfer in the form of bond energy transfer. When bond energy transfer occurs in specific environments, the energy state of the fluorophore changes, resulting in changes in fluorescence emission. TBET fluorescent probes can be used to detect molecular interactions, changes in molecular structure, and environmental changes inside cells. However, ICT fluorescent probes have diverse and highly sensitive characteristics for detecting biological elements through different mechanisms and regulation strategies. They have wide application potential in the field of biomedical imaging, drug delivery, and molecular recognition and are expected to play important roles in biomedical research and clinical diagnostics.
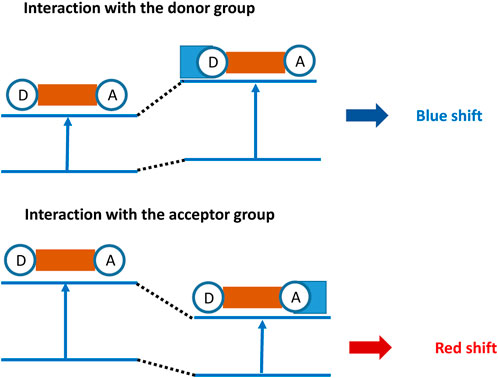
ICT-based fluorescent probes have a donor–acceptor structure, which are connected together through conjugated function, forming an electron system with the push–pull effect (Jana et al., 2021; Huang et al., 2023). This contributes to the charge in the whole dye system and shows a certain distribution structure. Thus, the formed ground-state electron cloud in the dye determines the fluorescence spectrum performance. When the fluorescent probe undergoes coordination with the analyte, the energy gap between the HOMO and LUMO orbitals of the fluorophore is altered. Due to the different binding affinities between the analyte and the fluorescent probe, the wavelength can experience a redshift or blueshift (shown in Figure 1) (Peng et al., 2021). Redshift refers to the increased electron-withdrawing ability of the fluorophore upon binding to the analyte, leading to an increase in electron cloud density and a decrease in the energy gap between the HOMO and LUMO orbitals, resulting in a longer wavelength. Conversely, blueshift refers to a decrease in wavelength as a result of the electron-donating ability. These probes exhibit a shift in the spectrum wavelength, either redshift or blueshift, upon the addition of the analyte. The original fluorescence intensity decreases, while a new fluorescence intensity gradually increases. Therefore, by utilizing the ICT mechanism, ratio-based fluorescent probes can be constructed to achieve selective detection of the analyte (Jana et al., 2022).
Cells are the basic units that constitute living organisms and carry out life activities. They rely on intracellular pH and biological species such as various gases, metal ions, and anions to function normally in a highly complex and networked spatial environment in order to maintain normal cellular activities (Klymchenko, 2022). When these molecules exhibit an abnormal behavior, it can impair cell function and ultimately lead to the development of diseases in the organism (Pramanik and Das, 2021). For example, thiols play a very important role in the redox homeostasis within cells. Imbalance in the redox state can lead to changes in the cellular metabolism, including respiration. Hydrogen sulfide (H2S) is considered to participate in various physiological processes. It can be overexpressed in many cancer cells, and colon cancer cells can produce a large amount of H2S through excessive expression of cystathionine β-synthase (CBS). Therefore, high-sensitivity detection of small molecules and biomacromolecules in cells is of great significance for the understanding of the molecular-level cellular functions and the mechanism underlying disease development. Herein, we reviewed the recent developments of ICT probes focusing primarily on the detection of intracellular pH, various gases (H2S, CO, H2O2, and NO), metal ions (Cu2+, Hg2+, Pb2+, Zn2+, and Al3+), and anions (ClO−, CN−, SO32−, and F−). Additionally, the issues and limitations of ICT-based fluorescent probes for in vivo detection were discussed, and the clinical translational potential was explored.
Intracellular pH detection
The intracellular pH (pHi) plays an irreplaceable role in maintaining various metabolic processes in cells. Enzyme activity, signal transduction, and other cellular functions are closely related to pHi. Studies have shown that various diseases, including cancer, Alzheimer’s disease, and Down syndrome, are closely associated with abnormal pHi in cells. Therefore, real-time, accurate, and sensitive monitoring of intracellular pHi changes reveals important information for studying the pathological and physiological processes related to cellular pHi. Small-molecule fluorescent probes used for measuring the pH of specific compartments within cells usually require the following characteristics: 1) the probe must have the ability to target the specific compartment; 2) the linear response range of the probe must include the pH value of the compartment, and its pKa should be as close as possible to the pH value of the compartment to achieve maximum sensitivity; 3) the probe should have a strong anti-interference ability against various cations, amino acids, and other biomolecules present in the cell; 4) reversibility is necessary for the real-time monitoring of pH fluctuations using the probe; and 5) the probe should have low toxicity to cells, excellent membrane permeability, and leak-proof ability. For instance, a ratio-type fluorescent probe, benzo (1,2-b:4,5-b′)dithiophene (BTDB), with a large Stokes shift was prepared based on the ICT luminescence mechanism (Zhang et al., 2016b). BTDB emits at 425 nm under alkaline conditions. As the pH decreases, the N on the aniline group becomes protonated, which enhanced the ICT effect and made the emission band gradually shift to 595 nm (a Stokes shift of 177 nm), presenting excellent lysosome-targeting ability in cells. In another research, a benzoindole-based colorimetric and naked-eye hemicyanine fluorescent probe 2,3-trimethy-3-[2,4-(dihyoxyl-4-yl)vinyl]-3H-benzo[e]indole (BiDD) was obtained for pH detection within the range of 4.4–6.2. In addition, BiDD also possessed high sensitivity and selectivity and low cytotoxicity in HeLa cells when the fluctuations in pH were visualized (Zhang et al., 2020).
Gas detection
Normally, abnormal concentrations of cellular gases such as H2S, CO, H2O2, NO, and SO2 always lead to serious diseases, including ischemia–reperfusion injury, vasodilation, cell death, angiogenesis, neural regulation, inflammation therapy, insulin signaling, and gas stress response (Liu et al., 2023a). Niu et al. (2022) introduced nitrobenzoxadiazole (NBD) ether and NBD amine as thiol recognition sites into the fluorescent probe for H2S detection as low as 81.1 nM. Excessive CO can cause oxygen deprivation in organisms, even posing a life-threatening risk. For CO detection, the fluorescent group naphthalimide, based on nitro-to-amino conversion, showed dazzling green fluorescence (Zhang et al., 2021). Furthermore, biological imaging experiments demonstrated that the ICT-based probes could control the changes in exogenous/endogenous CO in living cells. Excessive H2O2 can induce inflammatory responses, impair the microenvironment of tissue regeneration, and hinder wound healing. Li et al. (2022) first fabricated a naphthalimide−triphenylamine-based probe for H2O2 imaging with a low concentration of 44 nM and a quick reaction time (15 min) to H2O2 upon ICT effect in HepG2 cells (shown in Figure 2). NO participates in various physiological and pathological processes, whose homeostasis is related to cardiovascular diseases. A dicarboximide anthracene-based fluorescent probe was constructed to detect the NO level in living cells, showing an excellent sensitivity (5.52 nM) to NO in zebrafish (Wang et al., 2022). In addition, epidemiological research proved that inhalation of SO2 can harm the nervous system through destroyed redox balance in cells. Liu et al. (2023b) reported a dual-site multifunctional coumarin fluorescent probe for H2O2 and SO2 (derivatives SO32−) detection in vitro and in vivo.
Metal ion detection
Metal ions, including Cu2+, Hg2+, Pb2+, Zn2+, and Al3+, can cause chronic poisoning when they accumulate to a certain concentration (Alizadeh et al., 2020). They can accumulate thousands of times in organisms, causing significant harm to the nervous system, digestive system, immune system, kidneys, and liver. In addition, toxic metal ions can accumulate in the body and cause harm to living organisms even at low concentrations (Alizadeh et al., 2020; Zhao et al., 2023). In addition, heavy metal ions can affect the activity of DNA and proteins, causing chronic toxicity. Therefore, exploring a sensitive and rapid method for detecting heavy metal ions is of great significance and urgency for human safety. Hence, an ICT-based fluorescent probe (E)− 2-{3-[4-(diethylamino)phenyl]acryloyl}phenylpyridinecarboxylate (DPAP) was designed and obtained for the specific detection of Cu2+, whose detection limit was as low as 15.2 nM with low cell toxicity and good permeability (Liu et al., 2023c). In another work, a malononitrile isophorone-based probe was fabricated to detect Hg2+ rapidly with low biological toxicity. It also possessed a larger Stokes shift and a pronounced UV-vis absorption redshift, suggesting the great potential of Hg2+ detection in living cells (Pei et al., 2023). Moreover, (E)-N 0 -{[2-(4′-(diphenylamino)-(1,1′-biphenyl)-4-yl)-2H-1,2,3-triazol-4-yl]methylene} (DBTBH) was synthesized for Pb2+ detection with a limit concentration of 4.49 × 10−8 M in HeLa cells, showing a promising platform for biological applications (Xue et al., 2021). In addition, a dual fluorescence probe 7-hydroxy-8-{[(2-(hydroxymethyl)quinolin-8-yl)imino]methyl}-coumarin (XL), which consists of formylcoumarin and aminoquinoline moieties, was obtained for Zn2+ and Al3+ ion detection in PC12 cells, with detection limits of 3.75 × 10−8 and 1.14 × 10−8 M, respectively (Fu et al., 2020).
Anion detection
Anions in cells such as ClO−, CN−, SO32−, and F− play important roles in cell proliferation, cell differentiation, immune response, energy conversion, and signal transduction (Zheng et al., 2023). They exhibit high reactivity and can directly modify various biomolecules through oxidation, nitration, and halogenation. Recently, a ClO− probe was reported, which consists of N-alkyl-1,8-naphthalimide-4-boronate ester (NPI) and rhodamine B linked by ethylenediamine. Then, the boronate ester was grafted to the compound to react with HClO at 1.36 nM (Zhu et al., 2023). Next, a CN− probe was designed and synthesized based on a fluorene group and a hemicyanine group, which were linked by a conjugated linker. The probe was found to present rapid reaction, high sensitivity, and selectivity with CN− (Li et al., 2018). In addition, a benzimidazole-based fluorescence probe was synthesized for the detection of SO32−. Prior to the addition of SO32−, the probe is excited by light, and the electrons from the electron-donating benzimidazole moiety are transferred to the electron-accepting aldehyde moiety, resulting in an ICT effect. However, upon the addition of Na2SO3, the probe undergoes a nucleophilic addition reaction, inhibiting the ICT effect and making the green fluorescence change to blue. This process was certificated by mass spectrometry, hydrogen spectroscopy titration, and theoretical calculations. Finally, the probe was employed for the sulfite detection in HepG2 cells (Zhang et al., 2016a). Last but important is the F− fluorescent probe which utilizes an F-triggered specific demethylation reaction to induce the opening of the intramolecular charge transfer (ICT) interaction between the donor phenolate anion on IS-NR-O and the acceptor acetonitrile, thereby achieving a dual colorimetric and fluorescent response to fluoride ions with excellent selectivity. Furthermore, the probe exhibits a good linear relationship with fluoride ions over a wide concentration range of 0.38–6.84 ppm and a low detection limit of 0.09 ppm (Hu et al., 2019).
Conclusion
Recently, various fluorescent probes based on ICT have been designed and gained for intracellular pH and biological species detection, which was believed to promote the developments in applications of biomedicine. In summary, through the design and modification of ICT fluorescent probes, biological targets can be detected accurately. Ratio-type fluorescent probes with a large Stokes shift can dynamically monitor changes in intracellular pH in real time. By designing the D-A structure, different types of ICT fluorescent probes can be used for the rapid detection of various gases, heavy metal ions, and anions in living organisms at the nM level.
However, there are still several aspects that need to be further explored. These include the following: 1. High sensitivity. Reducing the effective concentration threshold of the fluorescent probe improves its sensitivity, which was at lower concentrations of reactive groups, resulting in better detection results. 2. Specificity and selectivity. Fluorescent probes should be able to recognize and bind to target molecules or pathological changes while not interacting non-specifically with other molecules or cells. It is challenging to improve the specific detection of fluorescent probes and reduce non-specific signal interference to obtain better imaging results. It is also promising to cleverly design and synthesize probes to achieve the detection of multiple biological activities with a single probe, thereby achieving multiple detection biological species. 3. Biocompatibility. It is a basic requirement to ensure the safety to the human body and compatibility with biological tissues in the conversion process of fluorescent probes. Fluorescent probes must have low toxicity, low immunogenicity, and good biocompatibility in order to be safely used in clinical applications. The metabolic pathways and toxicity of synthesized molecular compounds must be clarified clearly in organisms, especially for probes that are bound to high-molecular-weight polymers. The metabolic pathways of degradation products and inflammatory reactions also need to be investigated in organs such as the heart, liver, spleen, lungs, and kidneys. 4. Cell permeability. Fluorescent probes should own the ability to pass through the cell membrane and reach target cells or tissues. This requires fluorescent probes to have appropriate chemical properties and size in order to effectively penetrate and distribute in the organism. 5. Integrated diagnosis and therapy. It is a promising solution to integrate the detection of fluorescent probes with treatment by combining them with drugs or nanomedicine delivery systems, to achieve integrated diagnosis and therapy.
More importantly, how to translate laboratory findings into clinical applications and successfully achieve the clinical translation of fluorescent probes is a challenging task that needs to be urgently addressed. Moreover, fluorescent probes need to maintain satisfying stability in the in vivo environment, unaffected by factors such as light, acidity or alkalinity, and temperature. This is crucial for achieving reliable fluorescent signals in clinical applications. In addition, fluorescent probes must have good optical properties such as high fluorescence quantum yield, long fluorescence lifetime, and high photostability. This can enhance the intensity and stability of fluorescent signals, making them more suitable for medical imaging or diagnostics. Effectively labeling fluorescent probes onto suitable carriers or nanoparticles and delivering them to target cells or tissues is also a challenging task. More importantly, converting fluorescent probes into clinical applications requires conducting clinical trials to validate their safety and efficacy. Additionally, they need to comply with relevant regulatory requirements and regulations. These challenges require continuous research and development to drive the translation of fluorescent probes from the laboratory to the clinic. Interdisciplinary collaboration and technological innovation will play a crucial role in overcoming these challenges.
Author contributions
YoW: writing–original draft. CG and YZ: writing–original draft and investigation. YaW: writing–review and editing. DZ: funding acquisition, supervision, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82101457) and the Shandong Provincial Natural Science Foundation of China (ZR2021QH218).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alizadeh, T., Sharifi, A. R., and Ganjali, M. R. (2020). A new bio-compatible Cd2+-selective nanostructured fluorescent imprinted polymer for cadmium ion sensing in aqueous media and its application in bio imaging in Vero cells. RSC Adv. 10, 4110–4117. doi:10.1039/c9ra06910k
Danylchuk, D. I., Jouard, P.-H., and Klymchenko, A. S. (2021). Targeted solvatochromic fluorescent probes for imaging lipid order in organelles under oxidative and mechanical stress. J. Am. Chem. Soc. 143, 912–924. doi:10.1021/jacs.0c10972
Fang, H., Chen, Y., Jiang, Z., He, W., and Guo, Z. (2023). Fluorescent probes for biological species and microenvironments: from rational design to bioimaging applications. Accounts Chem. Res. 56, 258–269. doi:10.1021/acs.accounts.2c00643
Fu, J., Chang, Y., Li, B., Wang, X., Xie, X., and Xu, K. (2020). A dual fluorescence probe for Zn2+ and Al3+ through differentially response and bioimaging in living cells. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 225, 117493. doi:10.1016/j.saa.2019.117493
Hu, Q., Huang, Q., Mao, Y., Liu, X., Tan, F., Wang, Y., et al. (2019). A near-infrared large Stokes shift probe based enhanced ICT strategy for F- detection in real samples and cell imaging. Tetrahedron 75, 130762. doi:10.1016/j.tet.2019.130762
Huang, J., Zhou, Y., Wang, W., Zhu, J., Li, X., Fang, M., et al. (2023). A fluorescent probe based on triphenylamine with AIE and ICT characteristics for hydrazine detection. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 286, 122011. doi:10.1016/j.saa.2022.122011
Jana, A., Baruah, M., Munan, S., and Samanta, A. (2021). ICT based water-soluble fluorescent probe for discriminating mono and dicarbonyl species and analysis in foods. Chem. Commun. 57, 6380–6383. doi:10.1039/d1cc02600c
Jana, A., Baruah, M., and Samanta, A. (2022). Activity-based fluorescent probes for sensing and imaging of reactive carbonyl species (RCSs). Chem. – Asian J. 17, e202200044. doi:10.1002/asia.202200044
Klymchenko, A. S. (2022). Fluorescent probes for lipid membranes: from the cell surface to organelles. Accounts Chem. Res. 56, 1–12. doi:10.1021/acs.accounts.2c00586
Li, L., Zan, M., Qie, X., Miao, P., Yue, J., Chang, Z., et al. (2018). A highly selective fluorescent probe for cyanide ion and its detection mechanism from theoretical calculations. Talanta 185, 1–6. doi:10.1016/j.talanta.2018.03.013
Li, M., Wang, B., Liu, J., Zhang, Z., Chen, L., Li, Y., et al. (2022). Lipid droplet-specific dual-response fluorescent probe for the detection of polarity and H2O2 and its application in living cells. Anal. Chem. 94, 9732–9739. doi:10.1021/acs.analchem.2c01243
Liu, D., Hessler, W., and Henary, M. (2023a). H2S sensors: synthesis, optical properties, and selected biomedical applications under visible and NIR light. Molecules 28, 1295. doi:10.3390/molecules28031295
Liu, X., Shi, T., Xu, C., Zhu, M., and Wang, Y. (2023c). A highly selective and sensitive ICT-based Cu2+ fluorescent probe and its application in bioimaging. Ecotoxicol. Environ. Saf. 262, 115127. doi:10.1016/j.ecoenv.2023.115127
Liu, X.-L., Yan, M., Chen, Z.-G., Zhang, B., Yao, N., Zhao, S., et al. (2023b). A dual-site multifunctional fluorescent probe for selective detection of endogenous H2O2 and SO2 derivatives based on ICT process and its bioimaging application. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 286, 121955. doi:10.1016/j.saa.2022.121955
Niu, H., Duan, Y., Zhang, Y., Hua, X., Xu, C., Li, Z., et al. (2022). A bifunctional fluorescent probe based on PET & ICT for simultaneously recognizing Cys and H2S in living cells. J. Photochem. Photobiol. B Biol. 230, 112441. doi:10.1016/j.jphotobiol.2022.112441
Pei, S., Li, C., Pei, X., Zhang, X., Chi, Y., Zeng, W., et al. (2023). A fluorescent probe based on an enhanced ICT effect for Hg2+ detection and cell imaging. Anal. Methods 15, 3026–3033. doi:10.1039/d3ay00544e
Peng, R., Luo, Y., Yao, C., Cui, Q., Wu, Q., and Li, L. (2021). Intramolecular charge transfer-based conjugated oligomer with fluorescence, efficient photodynamics, and photothermal activities. ACS Appl. Bio Mater. 4, 6565–6574. doi:10.1021/acsabm.1c00719
Wang, L., Wang, Z., Chen, Y., Huang, Z., Huang, X., Xue, M., et al. (2022). A novel dual-channel fluorescent probe for selectively and sensitively imaging endogenous nitric oxide in living cells and zebrafish. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 277, 121280. doi:10.1016/j.saa.2022.121280
Xue, S., Xie, Z., Chu, Y., Shi, W., Liu, Y., and Zhao, Y. (2021). Highly selective and sensitive fluorescent probe possessing AIEE and ICT properties for rapid detection of Pb2+ in aqueous medium and its applications in living cells. Luminescence 37, 108–117. doi:10.1002/bio.4151
Zhang, L.-J., Wang, Z.-Y., Cao, X.-J., Liu, J.-T., and Zhao, B.-X. (2016a). An effective ICT-based and ratiometric fluorescent probe for sensing sulfite. Sensors Actuators B Chem. 236, 741–748. doi:10.1016/j.snb.2016.06.055
Zhang, W.-J., Fan, L., Li, Z.-B., Ou, T., Zhai, H.-J., Yang, J., et al. (2016b). Thiazole-based ratiometric fluorescence pH probe with large Stokes shift for intracellular imaging. Sensors Actuators B Chem. 233, 566–573. doi:10.1016/j.snb.2016.04.122
Zhang, Y., Bu, F., Zhao, Y., Zhao, B., Wang, L., and Song, B. (2020). A hemicyanine fluorescent probe with intramolecular charge transfer (ICT) mechanism for highly sensitive and selective detection of acidic pH and its application in living cells. Anal. Chim. Acta 1098, 155–163. doi:10.1016/j.aca.2019.11.040
Zhang, Y., Tang, Y., Kong, X., and Lin, W. (2021). An endoplasmic reticulum targetable turn-on fluorescence probe for imaging application of carbon monoxide in living cells. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 247, 119150. doi:10.1016/j.saa.2020.119150
Zhao, J., Lian, J., Pan, W., Du, J., Liu, Z., and Zhao, L. (2023). A bifunctional fluorescence probe based on AIE-ICT strategy for visual detection of Cu2+/Co2+ in complex matrix. Molecules 28, 2059. doi:10.3390/molecules28052059
Zheng, Y., Wu, S., Bing, Y., Li, H., Liu, X., Li, W., et al. (2023). A simple ICT-based fluorescent probe for HOCl and bioimaging applications. Biosensors 13, 744. doi:10.3390/bios13070744





Add Comment