Symbiont prevalence in Ixodes ricinus
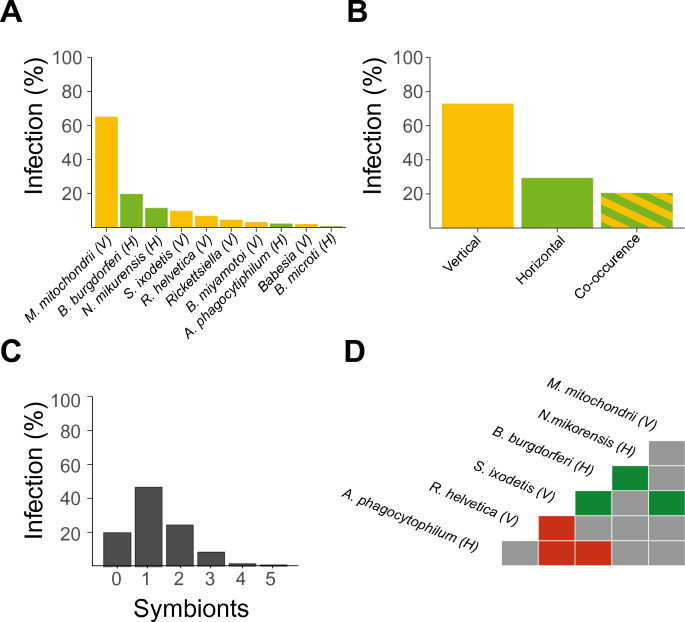
A total of 1500 ticks were collected and tested for the presence of 10 different symbiont species (Table 2, Fig. 1A). The vertically transmitted M. mitochondrii and horizontally transmitted B. burgdorferi s.l. were the most abundant symbiont species in the I. ricinus nymphs with an infection prevalence of 65.5% and 19.8% respectively.
A Infection percentage of individual ticks with each of the 10 symbiont species. B Infection percentage of either vertically transmitted symbionts, horizontally transmitted symbionts or co-infection with a horizontally and a vertically transmitted symbiont. C Infection percentages of the number of horizontally and vertically transmitted symbionts combined per nymph. D Co-occurrence matrix of symbionts. Only significant co-occurrences are shown. Green and red colours indicate significant (P < 0.05) positive or negative co-occurrence of the symbiont species in ticks, respectively. The transmission mode is depicted as (V) vertically or (H) horizontally
At least one vertically transmitted symbiont species was present in 72.9% of the collected nymphal ticks, whereas horizontally transmitted symbiont species were present in 29.3% of the nymphs (Fig. 1B). Vertically and horizontally transmitted symbionts co-occurred in 21.8% of the nymphs (Fig. 1B). Of all the nymphs tested, 0.1% were infected with five symbiont species, 1.4% were infected with four symbiont species, 8.2% were infected with three symbiont species, 24.2% were infected with two symbiont species, and approximately half of the nymphs (46.4%) were infected with one symbiont species (Fig. 1C). About one in five (19.7%) nymphs was not infected with any of the ten symbiont species for which we tested.
Significant negative co-occurrences were observed for A. phagocytophilum with S. ixodetis and B. burgdorferi s.l., and for R. helvetica with S. ixodetis (Fig. 1D, Additional file 1: Table S1). In addition, positive co-occurrences were observed for B. burgdorferi s.l. with N. mikurensis and for S. ixodetis with M. mitochondrii and with B. burgdorferi s.l. No significant co-occurrences were observed for the other symbiont-symbiont combinations.
Tick weight and lipid fraction
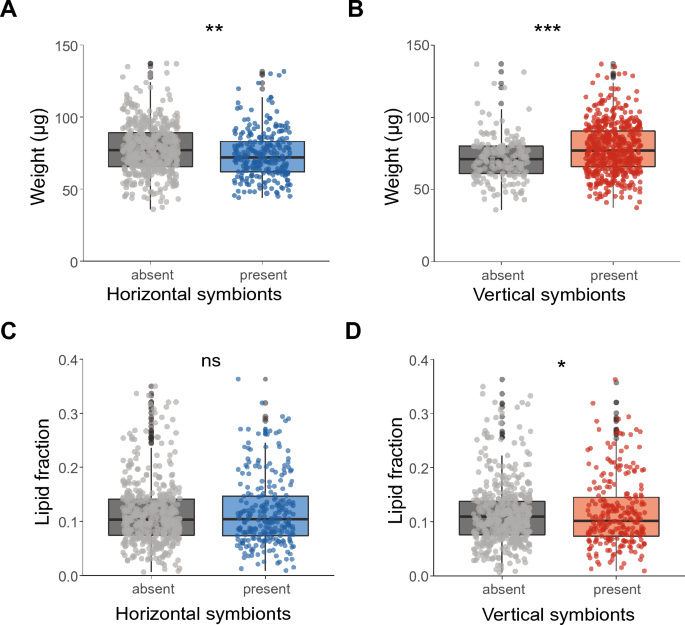
For the first group of individual ticks, the weight and lipid fraction of ticks were determined to study the associations between the presence or absence of symbiont species and tick physiology. When symbiont species were pooled by their transmission mode, the presence of the vertically transmitted symbionts was associated with a higher median body weight of ticks of 8.5% (GLMM, χ2 = 28.1, df = 1, P < 0.001, Fig. 2B), which is in agreement with our hypothesis. In contrast, the presence of the horizontally transmitted symbionts was associated with a 7% lower median body weight of ticks (GLMM, χ2 = 9.0, df = 1, P < 0.01, Fig. 2A). In general, ticks with a higher lipid fraction were also heavier (GLMM, χ2 = 16.9, df = 1, P < 0.001). The presence of horizontally transmitted symbionts was not associated with the lipid fraction of ticks (GLMM, χ2 = 2.4, df = 1, P > 0.05, Fig. 2C), whereas the presence of vertically transmitted symbionts was associated with a reduced median lipid fraction of 7.6% of the ticks (GLMM, χ2 = 4.5, df = 1, P < 0.05, Fig. 2D), which contradicts our hypothesis. We observed a significant random effect of day on which the ticks were collected for all (G)LMMs used.
Associations between weight or lipid fraction and the presence or absence of horizontally and vertically transmitted symbiont species. A Weight (µg) of ticks with or without horizontally transmitted symbionts. B Weight (µg) of ticks with or without vertically transmitted symbionts. C Lipid fraction of ticks with or without horizontally transmitted symbionts. D Lipid fraction of ticks with or without vertically transmitted symbionts. The lipid fraction was calculated by dividing the lipid weight by the tick dry weight. GLM: ns = not significant, *P < 0.05, **P < 0.01, ***P < 0.001
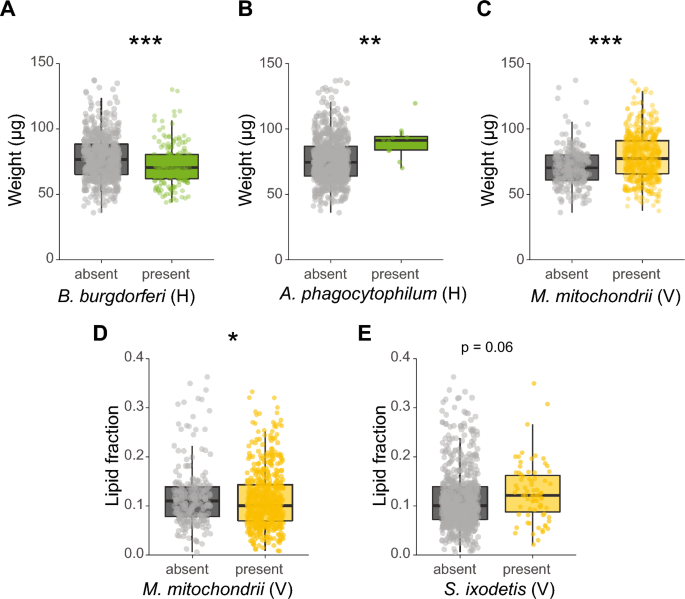
At the individual symbiont species level, two horizontally transmitted symbionts were significantly associated with the weight of ticks: B. burgdorferi s.l.-infected ticks had an 8.8% lower median body weight (GLMM, χ2 = 12.9, df = 1, P < 0.001, Fig. 3A), whereas A. phagocytophilum-infected ticks had a 22.5% higher median body weight (GLMM, χ2 = 7.2, P < 0.01, Fig. 3B) compared to uninfected ticks. Of note, infection with A. phagocytophilum was observed in 12 (1.6%) ticks, whereas infection with B. burgdorferi s.l. was observed in 176 (22.9%) ticks. Consistent with the analyses in which symbionts were pooled according to their transmission mode, no association was observed between the presence of horizontally transmitted symbiont species and the lipid fraction of nymphs.
Associations of weight or lipid fraction and the presence or absence of specific symbiont species. Only significant associations of weight or lipid fraction with symbionts are shown in the graphs. A Weight (µg) of Borrelia burgdorferi s.l. (un)-infected ticks. B Weight (µg) of Anaplasma phagocytophilum (un)-infected ticks. C Weight (µg) of Midichloria mitochondrii (un)-infected ticks. D Lipid fraction of M. mitochondrii (un)-infected ticks. E Lipid fraction of Spiroplasma ixodetis (un)-infected ticks. The lipid fraction was calculated by dividing the lipid weight by the tick dry weight. The transmission mode is depicted as (V) vertically or (H) horizontally. GLM: *P < 0.05, **P < 0.01, ***P < 0.001
Of the vertically transmitted symbiont species, only ticks infected with M. mitochondrii had a 10.3% higher median body weight (LRT, χ2 = 39.7, df = 1, P < 0.001, Fig. 3C). Furthermore, M. mitochondrii-infected ticks had a 9.9% lower median lipid fraction compared to uninfected ticks (GLMM, χ2 = 5.2, df = 1, P < 0.05, Fig. 3D). Ticks infected with the vertically transmitted S. ixodetis had a 19.8% higher median lipid fraction, but this difference was not significant (GLMM, χ2 = 3.4, df = 1, P = 0.06, Fig. 3E).
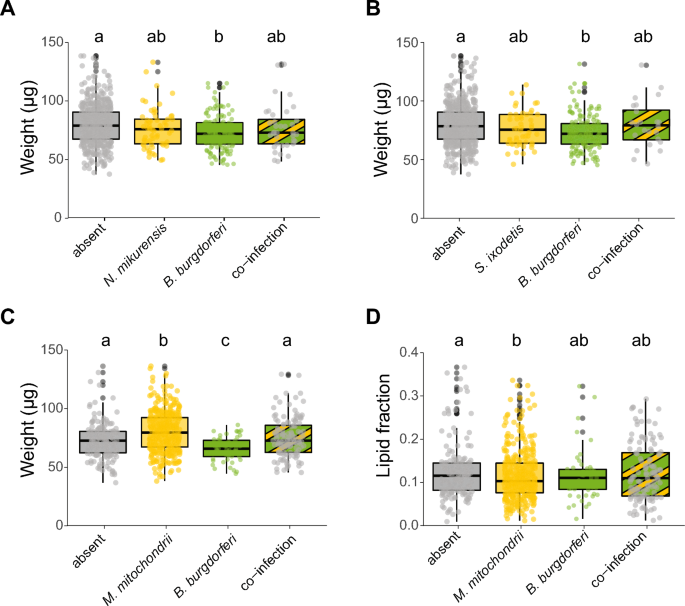
Symbiont-symbiont interactions of the four most prevalent symbionts were further analysed to study the associations of co-infection status with tick weight and lipid fraction. These analyses included the two most common vertically transmitted species, M. mitochondrii and S. ixodetis, and the two most common horizontally transmitted species, B. burgdorferi s.l. and N. mikurensis, in our study (Fig. 1A). When analysing the relation between symbionts and tick weight, a statistically significant interaction between B. burgdorferi s.l. and N. mikurensis was observed (GLMM, LRT, χ2 = 3.8, df = 1, P < 0.05, Fig. 4A). This was also the case for the interaction between B. burgdorferi s.l. and S. ixodetis (GLMM, LRT, χ2 = 3.9, df = 1, P < 0.05, Fig. 4B). In both analyses, ticks infected with B. burgdorferi s.l. had a significantly lower weight compared to uninfected ticks (Fig. 4A and B). However, the presence of N. mikurensis or S. ixodetis cancelled out the negative effect of B. burgdorferi s.l. on tick weight. No statistically significant interaction was observed between B. burgdorferi s.l. and M. mitochondrii (GLMM, LRT, χ2 = 0.7, df = 1, P > 0.05), but the lower weight of B. burgdorferi s.l.-infected ticks or the higher weight of M. mitochondrii-infected ticks was cancelled out in co-infected ticks (Fig. 4C).
Associations of weight or lipid fraction and the presence or absence of co-infections of specific symbionts. A Weight (µg) of ticks single infected or co-infected with Borrelia burgdorferi s.l. and Neoehrlichia mikurensis. B Weight (µg) of ticks single infected or co-infected with B. burgdorferi s.l. and Spiroplasma ixodetis. C Weight (µg) of ticks single infected or co-infected with B. burgdorferi s.l. and Midichloria mitochondrii. D Lipid fraction of ticks single infected or co-infected with B. burgdorferi s.l. and M. mitochondrii. The lipid fraction was calculated by dividing the lipid weight by the tick dry weight. Boxplots within each panel that have no letters in common are significantly different (GLM, emmeans, P < 0.05)
We further studied the associations between symbiont co-infection and the lipid fraction of ticks. A statistically significant interaction between M. mitochondrii and B. burgdorferi s.l. was observed (GLMM, LRT, χ2 = 5.1, df = 1, P < 0.05, Fig. 4D). Ticks infected only with M. mitochondrii had a significantly lower lipid fraction compared to uninfected ticks (Fig. 4D). However, the presence of B. burgdorferi s.l. in ticks cancelled out this association as co-infected ticks did not have significantly different lipid fractions compared to uninfected ticks.
Behavioural analysis of ticks
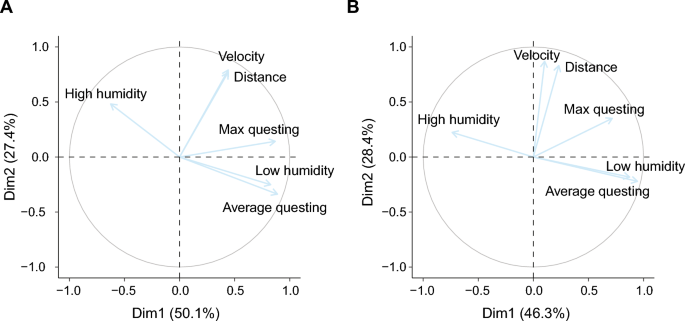
Analyses of the second group of ticks showed distinct activity patterns among the individual ticks that were observed in the behavioural assay (Fig. 5). These activity patterns were subsequently used to derive several behavioural parameters. PCA was used to analyse the behaviour of ticks based on these multiple, possibly collinear behavioural parameters (e.g. velocity, distance, questing height etc.). The principal components were then used to study the associations between these behavioural parameters and presence of symbiont species.
The six parameters associated with tick behaviour were reduced to two principal components, explaining respectively 50.1% and 27.4% of the variance (Table 3, Fig. 6A). The first component (PC1) axis scores were positively correlated with questing height (average questing height and maximum questing height) and the low humidity zone. The second component (PC2) axis scores were positively associated with walking distance and velocity and to a lesser extent with the high humidity zone.
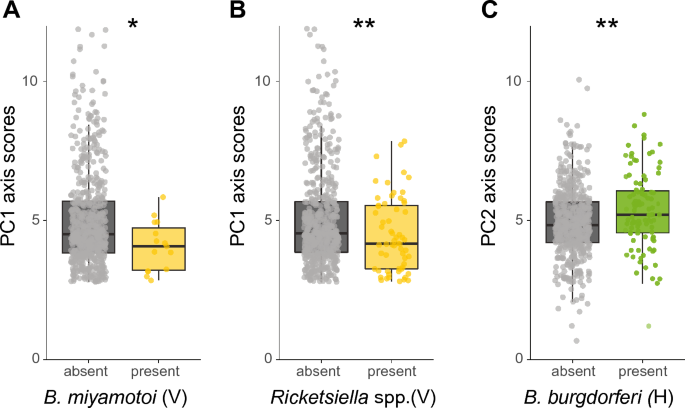
The presence or absence of horizontally or vertically transmitted symbionts was not associated with either the PC1 or PC2 axis scores (GLM, P > 0.05 for both variables, Additional file 1: Table S2). Nevertheless, we observed symbiont-specific associations for both principal components. For the vertically transmitted symbionts, B. miyamotoi and Rickettsiella spp., infected ticks had lower PC1 axis scores (GLMM χ2 = 5.4, df = 1, P < 0.05 and GLM, χ2 = 6.7, df = 1, P < 0.01, respectively, Fig. 7A and B), indicating that ticks infected with these two vertically transmitted symbionts quested lower and spent less time in the low humidity zone (located at the highest point in the assay) compared to uninfected ticks. Of the horizontally transmitted symbiont species, B. burgdorferi s.l. infected ticks had higher PC2 axis scores (GLM, χ2 = 7.3, df = 1, P < 0.01, Fig. 7C), indicating that ticks infected with B. burgdorferi s.l. moved further and faster compared to uninfected ticks. All other individual symbiont species were not significantly associated with either PC1 or PC2 axis scores. There was a significant effect of the date at which ticks were collected for both the PC1 and PC2 axis scores analyses. No significant interactions were observed between symbiont co-infections and PC1 or PC2 axis scores.
Associations of PC1 and PC2 axis scores with selected symbionts. A PC1 axis scores of Borrelia miyamotoi (un)-infected ticks. B PC1 axis scores of Rickettsiella spp. (un)-infected ticks. C PC2 axis scores of Borrelia burgdorferi s.l. (un)-infected ticks. The transmission mode is depicted as (V) vertically or (H) horizontally. GLM: *P < 0.05, **P < 0.01
Tick behaviour and physiology combined
A third group of 165 ticks was used to study the associations among tick behaviour, physiology and the presence of symbiont species within the same experiment. Also here, PCA reduced the behavioural parameters to two components, explaining 46.3% and 28.37% of the variance respectively (Table 4, Fig. 6B). Similar to the second experiment, the questing height and humidity zones strongly correlated to PC1 axis scores, and the distance and velocity parameters correlated to PC2 axis scores.
No associations were found between the presence of horizontally transmitted symbionts and PC1 axis scores. For PC2 axis scores, ticks infected with B. burgdorferi s.l. showed increased activity (distance moved, velocity) compared to uninfected ticks (GLM, χ2 = 4.1, df = 1, P < 0.05, Fig. 8A), as also observed in the second experiment. None of the vertically transmitted symbionts were associated with either score on PC1 or PC2 axes, as opposed to the previous experiment in which we observed associations of PC1 axis scores with B. miyamotoi and Rickettsiella spp.
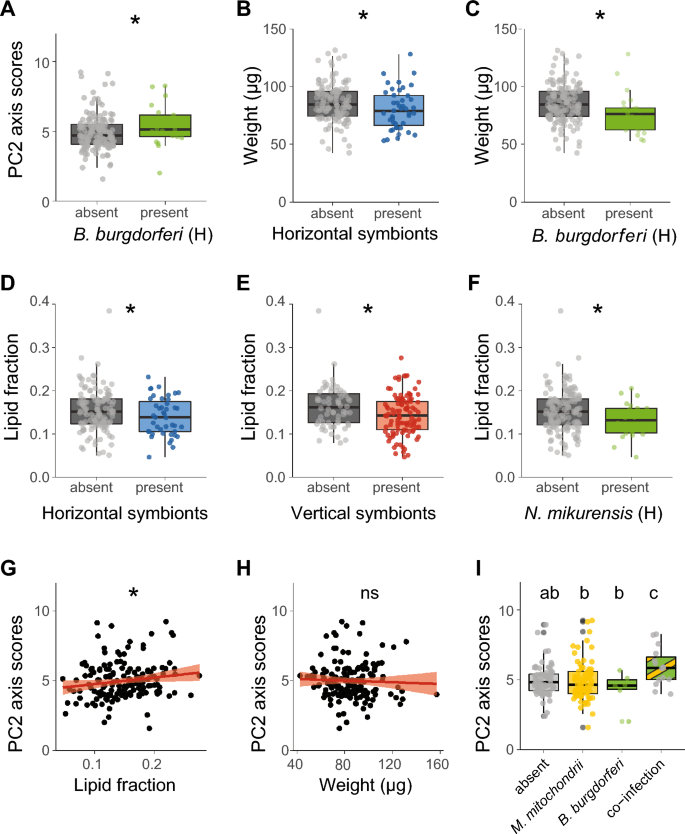
Associations among symbionts, behaviour and physiology of Ixodes ricinus nymphs. A PC2 axis scores of Borrelia burgdorferi s.l. (un)-infected ticks. B Weight (µg) of (un)-infected ticks with horizontally transmitted symbionts. C Weight (µg) of B. burgdorferi s.l. (un)-infected ticks. D Lipid fraction of (un)-infected ticks with horizontally transmitted symbionts. E Lipid fraction of (un)-infected ticks with vertically transmitted symbionts. F Lipid fraction of Neoehrlichia mikurensis (un)-infected ticks. G Association of PC2 axis scores with lipid fractions of ticks. The lipid fraction was expressed as the lipid weight divided by the tick dry weight. H Association of PC2 axis scores with tick weight (µg). I PC2 axis scores of ticks single infected or co-infected with B. burgdorferi s.l. and Midichloria mitochondrii. The lipid fraction was calculated by dividing the lipid weight by the tick dry weight. The horizontally transmission rmode is depicted as (H). GLM: ns = not significant, *P < 0.05. Boxplots within each panel that have no letters in common are significantly different (GLM, emmeans P < 0.05)
As we determined the weight and lipid fraction of ticks in this third experiment, we could also infer associations among symbiont species, tick physiology and tick behaviour. When symbionts were pooled by their transmission mode, we observed a negative association between the presence of horizontally transmitted symbionts and tick weight (GLM, χ2 = 5.0, df = 1, P < 0.05, Fig. 8B), as in the first experiment. Moreover, we also confirmed our previous findings at the symbiont species level that B. burgdorferi s.l.-infected ticks had a significantly lower weight compared to uninfected ticks (GLM, χ2 = 4. 8, df = 1, P < 0.05, Fig. 8C). The lipid fraction was lower in ticks infected with horizontally or vertically transmitted symbionts (GLM, χ2 = 4.2, df = 1, P < 0.05, GLM, χ2 = 4.1, df = 1, P < 0.05, respectively, Fig. 8D and E). At the symbiont species level, however, only ticks infected with N. mikurensis had a lower lipid fraction (GLM, χ2 = 4.8, df = 1, P < 0.05, Fig. 8F).
In contrast to the first two experiments, we could also investigate associations between tick weight and lipid fraction and the behavioural parameters (PC1 and PC2 scores) in the third experiment. The weight and lipid fraction of ticks was not associated with PC1 axis scores. The lipid fraction of ticks was positively associated with PC2 axis scores (χ2 = 5.9, df = 1, P < 0.05, Fig. 8G), whereas the weight of ticks was not associated with PC2 axis scores (Fig. 8H). This suggests that the distance travelled and walking velocity of ticks are positively associated with higher lipid fractions.
We further tested the associations of co-infections of the four most prevalent symbionts as described above. Of the six possible combinations, only the statistical interactions between B. burgdorferi s.l. and M. mitochondrii had significantly different PC2 axis scores (χ2 = 6.3, df = 1, P < 0.05, Fig. 8I). Specifically, co-infection of B. burgdorferi s.l. with M. mitochondrii was associated with increased PC2 axis scores compared to single infection with either symbiont or absence of both symbionts, suggesting that co-infection of these two symbionts increases the walking distance and velocity of ticks.
Summarizing all available results, we identified consistent associations between horizontally transmitted symbionts, in particular for B. burgdorferi s.l., with tick behaviour and physiology (Table 5). Infection with this symbiont was associated with reduced weight and increased activity in all experiments. For the vertically transmitted symbionts, we detected inconsistent associations with weight but consistent associations with lipid fraction (Table 5). More specifically, M. mitochondrii infection was associated with higher weight and reduced lipid fraction only in one of the two experiments. Symbiont-specific observations were observed for N. mikurensis, M. mitochondrii, A. phagocytophilum, B. miyamotoi and Rickettsiella spp. that were not consistent among experiments (Table 5). These discrepancies were most likely related to different sample sizes and small effect sizes of the observed associations.





Add Comment