Comparison of baseline data between the two groups
Differences in age, sex, pathological type, clinical stage, T stage, N stage, Epstein-Barr virus (EBV) DNA level, neutrophil-to-lymphocyte ratio (neutrophil/lymphocyte ratio), lactate dehydrogenase (LDH), HB, and EGFR expression were not statistically significant (all P > 0.05) (Table 1).
Comparison of short-term efficacy between the two groups
In the nimotuzumab group (52 cases), there were 48 and four cases of CR and PR, respectively, but no cases of SD and PD, with an objective remission rate of 100%. In the control group (57 patients), there were 45, 11, and one case(s) of CR, PR, and SD, respectively, but no cases of PD, with an objective remission rate of 98.2%. Compared with the complete remission rate of the two groups, the nimotuzumab group had a higher complete remission rate, the difference was statistically significant (χ2=3.876, P=0.049). One patient with SD in the control group had tumor-reduced (Table 2).
Comparison of long-term efficacy between the two groups
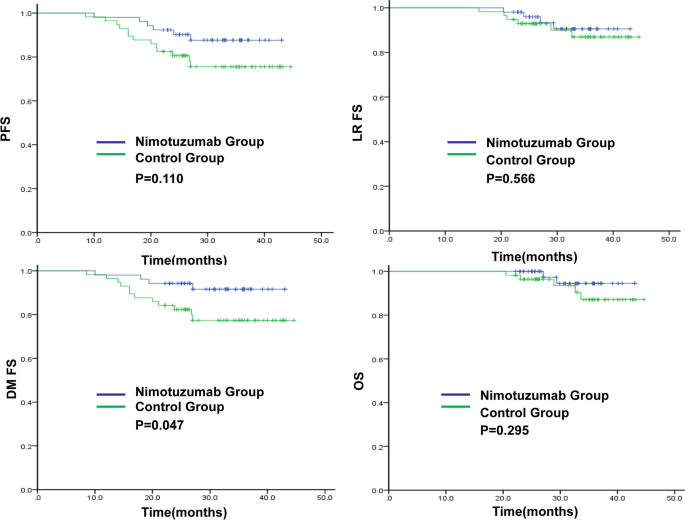
The 3-year DMFS rate in the nimotuzumab and control groups was 91.6% and 77.3%(P = 0.047), respectively.The 3-year PFS,LRFS and OS rates in the nimotuzumab and control groups were 87.6% vs 75.5% (P = 0.110), 90.5% vs 86.9% (P = 0.566) and 94.5% vs 87.1% (P = 0.295),respectively (Fig. 1). In the nimotuzumab group, there were six cases of disease progression, two of local recurrence, four of distant metastasis, and two of death. In the control group, there were 13 cases of disease progression, two of local recurrence, 12 of distant metastasis, and five cases of death.
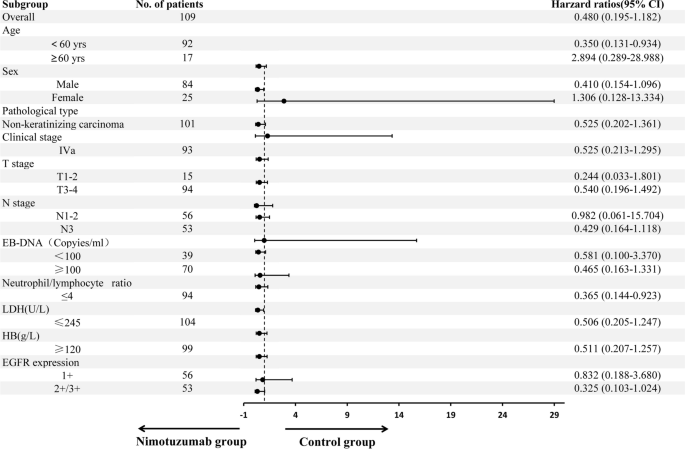
Subgroup analysis showed in patients with locally advanced NPC after neoadjuvant chemotherapy, concurrent chemoradiotherapy plus nimotuzumab targeted therapy had significant benefits to PFS for individuals aged < 60 years (hazard ratio [HR] = 0.350, 95% confidence interval [CI]: 0.131–0.934, P=0.036) and those with neutrophil/lymphocyte ratio ≤ 4 (HR = 0.365, 95% CI: 0.144–0.923, P = 0.033). There was a trend towards PFS benefit for patients with male (HR = 0.410, 95% CI: 0.154–1.096, P = 0.076),N3 (HR = 0.429, 95% CI: 0.164–1.118, P = 0.083), and those with EGFR (+ + / + + + + , HR = 0.325, 95% CI: 0.103–1.024, P = 0.055) (Fig. 2).
After radical chemoradiotherapy, EB DNA remained positive in four patients in the control group, while it was negative in the nimotuzumab group. The EB DNA levels after treatment,recent therapeutic effect and baseline factors were considered. Multivariate regression analysis showed that the neutrophil/lymphocyte ratio was an independent risk factor for disease progression (HR = 7.485, P = 0.012) and distant metastasis (HR = 17.540, P = 0.009) of locally advanced NPC, with a higher neutrophil/lymphocyte ratio being associated with an increased risk of disease progression and distant metastasis after treatment (Table 3).
Comparison of adverse reactions between the two groups
No grade 4 adverse reactions were observed in either group. Grade 3 oral mucosal reactions, as well as pharyngeal and esophageal reactions were slightly higher in the nimotuzumab group than in the control group, but the difference was not statistically significant. No significant differences were observed in the incidence of adverse reactions such as leukopenia, HB reduction, thrombocytopenia, elevated alanine aminotransferase (ALT), elevated aspartate aminotransferase (AST), elevated creatinine, nausea, vomiting, radiation dermatitis, weight loss, hyponatremia, hypokalemia, skin rash and infusion reaction between the two groups (P > 0.05) (Table 4).






Add Comment