Patients
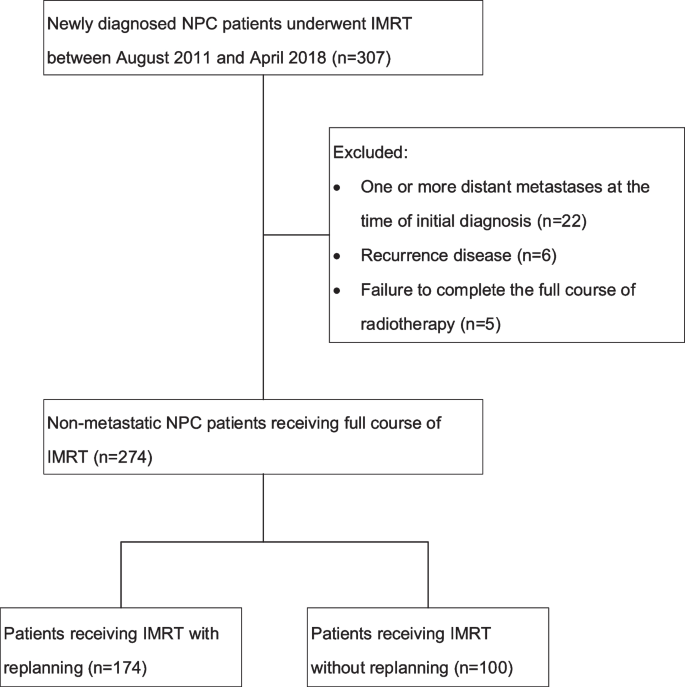
Our study enrolled consecutive patients diagnosed with NPC by histology and who had received IMRT at our medical center between August 2011 and April 2018. In total, 307 patients were identified, and 274 patients took part in the research. One hundred and seventy-nine patients who were treated between August 2011 and December 2015 were part of our previous study [12]. Patients with at least one metastatic disease at the time of first diagnosis (n = 22), recurrent disease (n = 6), and failure to accomplish the full course of IMRT (n = 5) were eliminated from the study. Figure 1 depicts the diagram for the research. Our medical center’s institutional review board authorized this study.
Chemotherapy
Patients diagnosed as stage I NPC received only IMRT, while those with stage II-IVa received concurrent chemotherapy. At the beginning of the study, adjuvant chemotherapy (AC) was routinely applied for patients with stage II-IVa NPC. From July 2016, induction chemotherapy (IC) gradually replaced AC as the conventional therapy strategy for patients with locally advanced NPC. There were two main AC and IC regimens, paclitaxel 135 mg/m2 on day 1, combined with cisplatin 25–30 mg/m2 throughout days 1–3 or docetaxel 75 mg/m2 on day 1, combined with cisplatin 25–30 mg/m2 throughout days 1–3, with intravenous infusion and 2–3 courses every 3 weeks. Two main concurrent chemotherapy regimens were also used, one was paclitaxel 35 mg/m2 as we previously reported [13], another was cisplatin 25–30 mg/m2, with infusions lasting three hours and five-six sessions administered weekly in conjunction with IMRT.
Intensity-modulated radiation therapy
All patients performed simulation CT scan from the skull vertex to 2 cm below the clavicles with a slice thickness of 2.5 mm within 2 days prior to IMRT. Eclipse (Version 10.0, Varian Medical Systems, Palo Alto, CA 94304, USA) and Pinnacle (Philips Radiation Oncology Systems, Milpitas, CA) treatment planning system were used to develop IMRT plans.
The GTVs of nasopharynx (GTVnx) and neck lymph nodes (GTVnd) were defined on the basis of magnetic resonance imaging (MRI) or CT scans, endoscopy, and clinical findings. For patients who received AC, the post chemotherapy volume of the primary lesion and the lymph nodes was used for GTVs delineation. The planning gross target volumes (PGTVs) including PGTVnx and PGTVnd were described as an additional 3 mm margin from GTVnx and GTVnd, respectively, to account for the errors of set-up and movement of the internal organ. The CTV1 was identified as the high-risk regions of tumor invasion and nodal involvement. The CTV2 was identified as the low-risk regions. The planning target volumes (PTVs) including PTV1 and PTV2 were described as an additional 3 mm margin from CTV1 and CTV2, respectively. The simultaneous integrated boost (SIB) technique was used, and the IMRT prescription was 70 to 76 Gy at 2.12 to 2.3 Gy to PGTVnx, 66 to 70 Gy at 2.0 to 2.12 Gy to PGTVnd, 60 to 66 Gy at 1.8 to 2.0 Gy to PTV1, and 56 to 60 Gy at 1.7 to 1.8 Gy to PTV2, in 33 fractions. For the purposes of optimization and evaluation, the RTOG 0225 protocol from the Radiation Therapy Oncology Group was employed as a standard constraint set [14].
Adaptive replanning
Prior to receiving treatment, patients were instructed to undergo adaptive replanning, with the detailed procedure, potential benefits (improved locoregional control, reduced toxicity), and the downsides (added expenses, prolonged treatment times) were particularly explained and the patients consented. Ultimately, patients who declined a second simulation CT scan during the course of IMRT were given IMRT without adaptive replanning, while other patients had IMRT with replanning. The re-simulation CT scan and replanning were carried out at the 15th and/or the 25th fraction of IMRT as we previously reported [15]. The replanning GTVs was described as residual shrunken tumor and positive lymph nodes. The replanning CTVs were outlined with the same extended margin from the corresponding GTVs and its involved regions were described as identical to the original plans with anatomical modifications. Tumor regression area was included in the replanning CTV1. The replanning PGTVs and PTVs were defined as 3 mm extensions from the replanning GTVs and CTVs respectively. Initial planning and replanning adhered to the same target prescription dose and dose limitation for essential structures.
Follow-up and data collection
All patients were observed one month after therapy, every three months for the first two years, every six months in the third to fifth years, and annually thereafter. Every subsequent appointment included a flexible fiberoptic endoscopy, head-and-neck scan, abdominal ultrasound, chest X-ray/CT, and blood tests. Electronic medical record system data on medical history, physical examination, blood tests, contrast-enhanced CT and MRI of the head and neck, chest CT, abdominal ultrasound, and bone emission computed tomography scans were obtained. All participants were restaged in accordance with the 8th staging system of the American Joint Committee on Cancer (AJCC). Locoregional failure was identified as a nasopharynx cancer recurrence and/or regional lymph node that was confirmed by biopsy or positron emission tomography/computed tomography (PET/CT). Vital status was determined using follow-up phone calls combined with medical records.
Failure pattern description
For locoregional recurrent patients, the MRI or CT images acquired at the time of locoregional recurrence were imported on the treatment planning systems. Image fusion of the recurrent scans with the previous simulation CT scans was performed based on bone landmarks. As for patients with replanning, the recurrent scans were integrated with the initial simulation CT scans and the re-simulation CT scans respectively. On the fused images, the recurrent GTVs of the primary site (rGTVnx) and neck lymph nodes (rGTVnd) were delineated layer by layer. The precise location of the recurrent GTVs was then assessed according to the fused images. If the recurrent GTVs were located outside GTVs of the re-simulation CT scans but inside the GTVs of the initial simulation CT scans, the location of the recurrences was defined as “shrunken area”. The type of recurrence was determined based on the 95% isodose lines. The last replans were used for dosimetric analyses in patients with replanning. If 95% of recurrent GTV was inside the 95% isodose, the pattern of recurrence was considered a “in-field” failure. If 20% to 95% of recurrent GTV was within the 95% isodose, the pattern of recurrence was considered a “marginal” failure. If less than 20% of recurrent GTV was inside the 95% isodose, the pattern of recurrence was considered a “out-field” failure [16].
Statistical analysis
Categorical variables could be represented by frequency and proportion of baseline attributes. Continuous variables could be represented by median and interquartile ranges (IQRs), while discrete variables may be represented by mean and standard deviations (SDs). The statistical analysis was performed using SPSS (IBM, Chicago, IL, version 22.0). Categorical variables were subjected to an X2 test. On continuous variables, a paired t-test was conducted to evaluate group variations. Survival rates were calculated from the date of initial treatment to the date of the event or the final follow-up visit. The estimates of survival rates were estimated using Kaplan–Meier method. The significant variation between survival curves were compared using the log-rank test. All tests of statistical significance were conducted using a two-sided distribution, and if the P value of the results was less than 0.05, they were considered significant.






Add Comment