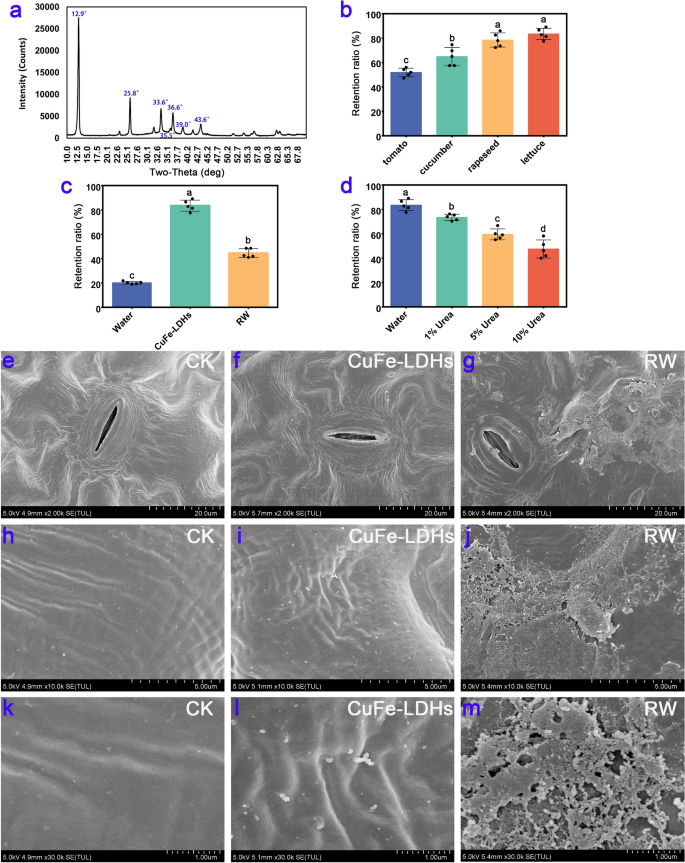
XRD results of CuFe-LDHs and its retention rate on the leaf surface
We conducted an XRD analysis on the synthesized CuFe-LDHs, and the results are shown in Fig. 1a. When the molar ratio of copper (Cu) to iron (Fe) was maintained at 2:1, distinct diffraction peaks corresponding to specific crystal planes of the layered structure, namely (003), (006), and (009), were observed at angles of 2θ = 12.9°, 25.8°, and 33.6°, respectively. These peaks exhibited well-defined profiles, indicating a high level of crystallinity. Additionally, the presence of the LDH structure was confirmed by the detection of diffraction peaks at 2θ = 36.6° and 43.6°, which can be attributed to the crystal planes of (015) and (018) [35]. Compared with the conventional LDH structure, the diffraction peaks of CuFe-LDHs were shifted upward at higher angles, primarily due to the Jahn–Teller effect induced by divalent copper ions, which results in structural distortion [36]. Furthermore, additional diffraction peaks at 2θ = 35.5° and 39.0° were observed, corresponding to the standard card (JCPDS: 48–1548), and this suggested the presence of a low quantity of monoclinic copper oxide (CuO) impurities in the synthesized material. Previous study showed that CuO NPs were more toxic than the Cu2+ [37]. Nevertheless, at low concentrations (< 2 mg/L), CuO NPs had no detrimental effect on plant growth [38]. Consequently, we can conclude that the presence of a low quantity of CuO impurities will not interfere the effects of CuFe-LDHs in plants. The TEM results, as shown in Additional file 1: Fig. S1, revealed that on the carbon film, CuFe-LDHs particle sizes range from approximately 30–100 nm. In order to confirm that the CuFe-LDHs dispersion are stable, zeta potential analyses are performed. The results, as shown in Additional file 1: Fig. S2, showed that the zeta potential of CuFe-LDHs dispersions was + 24.2 mV, suggesting that CuFe-LDHs are positive charged and the dispersions are relatively stable. As shown in Additional file 1: Fig. S3, the particle size of CuFe-LDHs was 60.1 nm. Both Zeta potential and particle size exhibit a normal distribution with a single peak, indicating that particles in the solution possess similar size and charge distributions, are uniformly dispersed in the solution, and exhibit high stability.
XRD results of CuFe-LDHs and their retention rate on the leaf surface. a XRD results of CuFe-LDHs. b The retention ratio on different plant leaves of 1 g/L CuFe-LDHs. c The retention ratio on the lettuce leaves of ddH2O, 1 g/L CuFe-LDHs, and 1 g/L RW. d The retention ratio of 1 g/L CuFe-LDHs on lettuce in different treatments. e–m SEM results of lettuce. CK, Control check. e, h, k CK, f, i, l 10 μg/mL CuFe-LDHs, g, j, m 10 μg/mL RW
To investigate whether LDH adheres to the leaves, we used simulated rainwater flushing to detect the retention of CuFe-LDHs on the leaf surface before and after rainwater flushing. After vertical flushing with 100 mL of ddH2O, the retention rates of CuFe-LDHs on the tomato, cucumber, rapeseed, and lettuce leaf surfaces were 51.9 ± 3.47%, 64.86 ± 7.53%, 78.48 ± 5.83%, and 83.48 ± 4.52%, respectively (Fig. 1b). The above results indicate that CuFe-LDHs show high retention on lettuce leaf surfaces. There were significant differences between the retention rates of CuFe-LDHs on the above leaf surfaces, ranging from 51.9 ± 3.47% to 83.48 ± 4.52%. The reason for this difference in retention rates can be attributed to leaf surface structure and waxes on leaf surfaces, which exhibit diverse characteristics among different species, classified as either weakly hydrophobic or strongly hydrophobic traits [39]. Consequently, this leads to varying retention rates when applying different species of leaves during spraying the same liquid [39, 40]. Comparison of the foliar retention of CuFe-LDHs and their raw materials on lettuce leaves revealed that the foliar retention of H2O, CuFe-LDHs, and their raw materials (RW) was 20.16 ± 1.11%, 83.48 ± 4.52%, and 44.52 ± 3.67%, respectively, after being washed with 100 mL of ddH2O (Fig. 1c). The retention of CuFe-LDHs on the surface of lettuce leaves far exceeded that of RW and water. To investigate the leaf surface adherence mechanism of CuFe-LDHs, LDHs were evenly applied to the leaves of lettuce. The leaves were washed with 100 mL of H2O and 1%, 5%, and 10% urea solutions, and the retention rates were 83.48 ± 4.52%, 73.48 ± 2.52%, 59.5 ± 4.47%, and 47.5 ± 7.36%, respectively (Fig. 1d). After gradient urea treatment, the retention rate of CuFe-LDHs on the surface of lettuce leaves decreased.
The adhesion phenomenon in nature is achieved via two mechanisms. (i) Adhesion is attained through the relative sliding at the contact interface facilitated by Van der Waals forces (e.g., geckos and spiders employ bristles on their feet to adhere to the contact surface). (ii) Adhesion is further strengthened at the contact interface by the secretion of substances that form hydrogen bonds (e.g., creeper plants secrete L-rhamnose to facilitate adhesion to the contact surface) [41]. Urea can destroy the hydrogen bonds inside protein molecules to promote their degradation [42]. After urea treatment, the retention of CuFe-LDHs was significantly reduced (Fig. 1d), indicating that the adsorption of LDHs on the leaf surface was determined by hydrogen bonding. The functional groups that can generate hydrogen bonds include hydroxy (-OH), amino (-NH3), carboxyl (-COOH), and carbonyl (C = O) [43]. As described in the “Experimental”, CuFe-LDHs were prepared using two types of nitrates and strongly alkaline NaOH, which introduced a large number of hydroxyl groups during the preparation process. The number of hydroxyl groups on the unit area has a certain relationship with adhesion [44], a large number of hydroxyl groups enriched on the surface of the material can mediate the adsorption of blades. The retention rate of RW is much lower than that of CuFe-LDHs. The most likely explanation is that RW is composed of copper nitrate and iron nitrate, which cannot bind to the leaf surface via hydrogen bonding. In short, CuFe-LDHs composed of Cu and Fe elements were compared with the synthetic raw material copper nitrate and iron nitrate, which enhance the adhesion of the leaf surface.
To observe the surface structure of the leaves after spraying, two-week-old lettuce leaves were sprayed with H2O, 10 μg/mL CuFe-LDHs, or 10 μg/mL RW; the SEM results are shown in Fig. 1e–m. It is widely known that most airborne dust particles carry a negative charge [45], which makes them prone to being adsorbed by positively charged particles. The retention of RW on the blade surface was lower compared with CuFe-LDHs (Fig. 1c), which might be responsible for the inability of most of the charged particles (Cu2+, Fe3+) in RW10 to bind to the blade surface. Instead, they might adsorb anions or airborne dust particles on the blade surface, forming aggregates (Fig. 1g, j, m). In contrast, LDHs formed electrically neutral particles and evenly dispersed on the surface of the blades (Fig. 1f, i, l). Therefore, by employing a comparatively lesser quantity of CuFe-LDHs, superior outcomes can be attained compared to those obtained with RW.
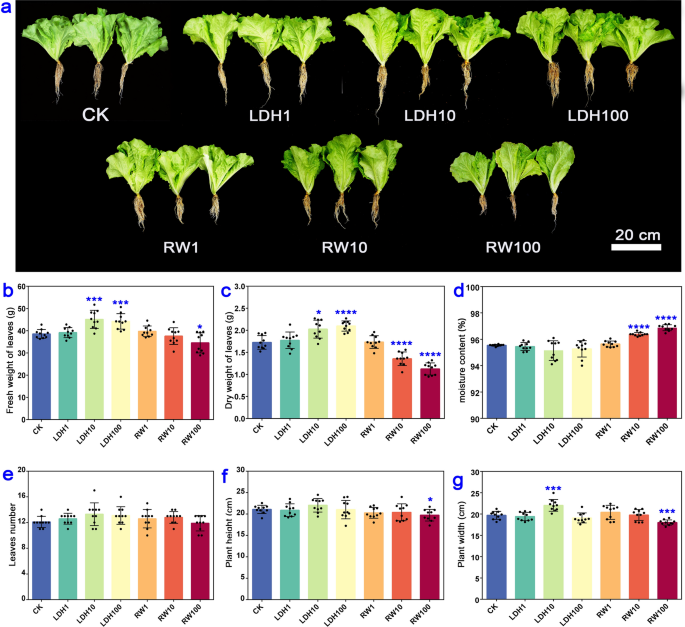
CuFe-LDHs promoted the growth of lettuce and affected the accumulation of elements one month after the initial spray
To investigate the effect of spraying different concentrations of CuFe-LDHs on plant growth, we took measurements of various parameters, including the fresh and dry leaf weight, moisture content, leaves number, plant height, and plant width one month after the initial spray. We also established a control group using RW, which contained the raw materials of CuFe-LDHs at an equivalent concentration. The phenotypic characteristics of lettuce were presented in Fig. 2a. The application of CuFe-LDHs at concentrations of 10 μg/mL (45.15 ± 3.84 g) and 100 μg/mL (44.23 ± 3.30 g) significantly increased the fresh weight of lettuce (p < 0.001) compared with the CK group (38.59 ± 1.88 g) (Fig. 2b). Conversely, the application of RW at 100 μg/mL had a significant inhibitory effect on the fresh weight of lettuce (p < 0.05) (Fig. 2b). Similarly, the dry weight of lettuce was significantly higher in the 10 μg/mL (2.03 ± 0.19 g) (p < 0.05) and 100 μg/mL (2.10 ± 0.11 g) (p < 0.0001) CuFe-LDHs groups compared with the CK group (1.73 ± 0.15 g) (Fig. 2c). In contrast, both 10 µg/mL (1.36 ± 0.14 g) and 100 μg/mL (1.13 ± 0.13 g) RW inhibited increases in the dry weight (p < 0.0001) (Fig. 2c). Moreover, the moisture content of lettuce was significantly higher in the 10 μg/mL (96.38 ± 0.13%) and 100 μg/mL (96.84 ± 0.20%) RW groups than in the CK group (95.55 ± 0.06%) (p < 0.0001) (Fig. 2d). Leaves number was unaffected by the application of CuFe-LDHs or RW (p > 0.05) (Fig. 2e). Plant height was significantly lower in the 100 μg/mL RW (19.73 ± 1.28 cm) group than in the CK group (21.09 ± 0.91 cm) (p < 0.05) (Fig. 2f). Plant width (22.03 ± 0.30 cm) was significantly higher in the 10 μg/mL CuFe-LDHs group than in the CK group (19.69 ± 0.90 cm), and the application of 100 μg/mL RW (18.02 ± 0.58 cm) resulted in a significant reduction in plant width (p < 0.001) (Fig. 2g). Overall, these findings demonstrate that the application of CuFe-LDHs at concentrations of 10–100 µg/mL promotes growth, whereas RW application at the same range of concentrations hinders growth. One month after the initial spray, LDH10 increased the Cu content in lettuce within an appropriate range (Table 1). In light of the substantial increases observed in the fresh weight, dry weight, and leaf width achieved by the spraying of 10 μg/mL CuFe-LDHs compared with 100 μg/mL LDH, 10 μg/mL CuFe-LDHs was considered the optimal concentration.
Effect of spraying different concentrations of CuFe-LDHs and RW on the phenotype of lettuce after one month. a Lettuce treated with varying concentrations of LDHs and RW. b Fresh weight of leaves. c Dry weight of leaves. d Moisture content. e Leaves number. f Plant height. g Plant width. Error bars represent SD. CuFe-LDHs = 1, 10, and 100 μg/mL correspond to LDH1, LDH10, and LDH100 for short. RW = 1, 10, and 100 μg/mL are RW1, RW10, and RW100 for short. *p < 0.05, ***p < 0.001, and ****p < 0.0001, Student’s t-test. Bar = 20 cm
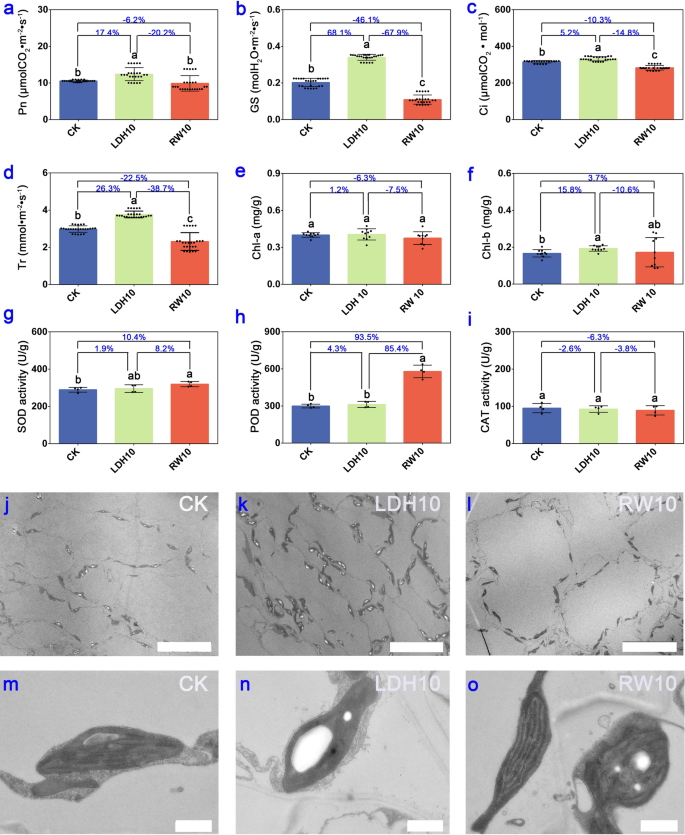
CuFe-LDHs enhance photosynthesis without affecting the antioxidant system and ultrastructure of lettuce leaves two weeks after the initial spray
To evaluate photosynthetic characteristics, antioxidant system, and ultrastructure, we utilized the lettuce plants treated with CK, LDH10, and RW10 two weeks after the initial application as experimental materials. Obviously, LDH10 and RW10 had a significant effect on photosynthesis after the initial spray for 2 weeks based on observed changes in multiple parameters, including net photosynthetic rate (Pn), stomatal conductance (GS), intercellular CO2 concentration (Ci), and transpiration rate (Tr) (p < 0.01) (Fig. 3a–d). These findings suggest that LDH10 significantly enhances photosynthesis in lettuce, whereas RW10 significantly inhibits photosynthesis. One of the factors contributing to this disparity is the uniform distribution of LDHs on the leaf surface, which allows them to bind to the leaves through hydrogen bonding (Fig. 1f, i, l). As a result, electrically neutral particles are formed that do not hinder stomatal function nor affect GS and Tr (Fig. 3b, d). Conversely, the physical shielding effect of RW10 hindered the photosynthetic reactions in lettuce leaves (Fig. 3a–d), which directly affected plant yield (Fig. 2b, c). Previous studies have highlighted the exceptional CO2 adsorption capability of LDHs, and this has made them extensively used in ongoing efforts to achieve carbon neutrality because of their high CO2 adsorption capacity [19]. In light of LDHs’ capacity to enhance the Pn and intercellular CO2 absorption (Fig. 3a, c), we hypothesize that the main mechanism underlying the adsorption of CO2 by LDHs stems from its ability to increase the Ci. This increase in Ci results in the augmentation of the substrate, thereby promoting an increase in the Pn.
Photosynthetic characteristics, antioxidant system, and ultrastructure of lettuce leaves two weeks after the initial spray of LDH10 and RW10. Net photosynthetic rate (Pn, a), stomatal conductance (GS, b), intercellular CO2 concentration (Ci, c), and transpiration rate (Tr, d) of lettuce. The chlorophyll a content (chl-a, e), chlorophyll b content (chl-b, f), and superoxide dismutase (SOD, g), peroxidase (POD, h), and catalase (CAT, i) activity in lettuce leaves following foliar exposure to LDH10 and RW10. Different lowercase letters indicate significant differences among treatments (p < 0.05). The percentages show the magnitude of change among the different treatment groups (LDH10/CK, RW10/CK, LDH10/RW10). Bar = 20 μm j, k, l. Bar = 1 μm m, n, o. The abbreviations used are the same as in Fig. 2
Furthermore, we speculate that the elevation of GS and Tr are related to the physiological activities of CuFe-LDHs after they enter plant cells. Previous studies have indicated that LDHs can overcome the barrier of the cell wall to enter plant cells [20, 25, 27, 29], and pass through the plasma membrane [25, 26]. Ultimately, LDHs undergoes decomposition in the elevated H+ environment within the cytoplasm or vacuole, slowly releasing the metal cations that make up the LDHs [46]. We can infer that CuFe-LDHs can enter cells and slowly degrade into low concentrations of Cu2+ and Fe3+ within cells, subsequently induce changes in the cellular physiological levels. Copper and iron are essential mineral elements for plant growth and development. Copper is a component of the photosynthetic electron transport chain, for CO2 assimilation and ATP synthesis [47], involved in photosynthetic reactions of PSII independent of plastocyanin [48]. Fe has greater importance in photosynthesis and respiration [49]. The photosynthetic machinery in plants is abundantly supplied with Fe atoms, comprising 12 Fe atoms per Photosystem I (PSI), 2 or 3 Fe atoms per Photosystem II (PSII), 5 Fe atoms within the cytochrome complex b6-f (cyt b6-f), and 2 iron atoms per ferredoxin molecule [50]. Therefore, photosynthetic organisms exhibit a high sensitivity to alterations in iron availability, leading to a significant reduction in photosynthetic activity when subjected to iron deficiency [51]. In summary, maintaining an appropriate concentration of Cu and Fe plays a pivotal role in enhancing the activities of PSI and PSII, facilitating CO2 assimilation, and promoting ATP synthesis. Moreover, within an optimal range, increasing iron content can elevate GS levels, thereby enhancing photosynthesis [52]. An increase in Fe content simultaneously enhances GS, which is consistent with our findings (Fig. 3b). In conclusion, we can infer that by gradually releasing low concentrations of Cu and Fe ions within cells, CuFe-LDHs influences the synthesis of proteins and enzymes related to photosynthesis, enhances GS and Pn, ultimately promoting photosynthesis.
No differences in the chlorophyll a content were observed among the LDH10, RW10, and CK treatments (Fig. 3e). LDH10 significantly increased the content of chlorophyll b; however, RW10 had no significant effect on the content of chlorophyll b (Fig. 3f). Chlorophyll a and its derivatives primarily absorb red light (620-700 nm), whereas chlorophyll b predominantly absorbs blue-violet light (400-500 nm) [53]. In light of the unique absorption spectrum of LDHs [54], we speculate that the presence of LDH10 on the leaf surface reduces the absorption of blue-violet light, thereby prompting the synthesis of chlorophyll b to enhance the absorption of blue-violet light.
Exposure to RW10 via foliage significantly increased the content of SOD (Fig. 3g) and POD (Fig. 3h) in lettuce leaves, which induced a stress response in the antioxidant system. However, there was no significant difference in the content of SOD (Fig. 3g), POD (Fig. 3h), or CAT (Fig. 3i) in lettuce in the LDH10 treatment, indicating that LDH10 does not induce stress responses in lettuce. The activity of antioxidant enzymes such as SOD, POD, and CAT plays a key role in scavenging excessive O2− and H2O2, thereby mitigating damage caused by biotic or abiotic stress [55]. SOD activity was 10.4% higher in RW10 than in the CK (Fig. 3g), and POD activity was 93.5% higher in RW10 than in the CK (Fig. 3h). These results indicate that SOD and POD effectively eliminate accumulated H2O2 in lettuce leaves, reducing the levels of free radicals and alleviating membrane lipid peroxidation damage in older leaves. Similarly, hydroponically cultured lettuce produces a substantial amount of SOD within its tissues when subjected to external stress, which enables them to scavenge stress-induced superoxide radicals and protect the plants [56]. Furthermore, Cd stress significantly up-regulates POD enzyme genes in lettuce, which enhances resistance to Cd stress [57].
Ultrastructural analysis revealed that the number of transient starch granules was significantly higher in leaves in the LDH10 treatment (Fig. 3k) than in the CK (Fig. 3j), and the number of transient starch granules was significantly lower in leaves in the RW10 treatment (Fig. 3l) than in the CK (Fig. 3j), suggesting that the application of LDH10 and RW10 may affect photosynthesis. In the lettuce leaf cells of CK, chloroplasts appeared elliptical, thylakoids were arranged parallel to the long axis of the chloroplasts, grana stacks formed granal lamellae, and the stroma was uniform (Fig. 3m). The intact chloroplasts in LDH10 leaves (Fig. 3n) had well-organized thylakoids and clear granaf lamellae, similar to the normal chloroplasts in CK. In contrast, analysis of RW10-treated leaves revealed disorganized stacks of chloroplasts and the presence of malformed chloroplasts (Fig. 3o). These findings suggest that the LDH10 treatment does not affect the structure of chloroplasts in lettuce leaves. To our surprise, LDH10 was not detected using the ultra-thin sectioning method (Fig. 3k, n). Previous studies have indicated that LDHs can overcome the barrier of the cell wall to enter plant cells [20, 25, 27, 29]. LDHs enter plant cells through the following steps: (1) smaller-sized LDHs can penetration across cell wall. Larger-sized LDHs need to undergo delamination into smaller-sized LDHs or nanosheets in the presence of CO2 and humidity to pass through the cell wall barrier [20, 27]; (2) LDHs pass through the plasma membrane via non-endocytic pathways and endocytosis [25]; (3) LDH undergoes decomposition in the elevated H+ environment within the cytoplasm or vacuole [25]. The exact mechanism behind this phenomenon has yet to be elucidated. The most plausible explanation is that LDH10 adsorbed onto the cell wall and slowly degraded into the cells due to CO2 and humidity, as described in previous studies [27, 29]. Another possibility that cannot be ruled out is that smaller layers of LDHs delaminated and entered the cells [25], but the LDH particles could not be observed via TEM because of their small size. Studies of duckweed have demonstrated that low concentrations of Cu2+ can promote plant growth, whereas high concentrations of Cu2+ can inhibit plant growth by disrupting the structure of chloroplasts or thylakoids and reducing the activity of photosystem II [58]. Interestingly, we observed damage to the chloroplasts and thylakoids in RW10 (Fig. 3o), but not in LDH10 (Fig. 3n). The most plausible explanation was that LDH10 contains Cu2+ both in its free form and bound to LDH layers, and it releases Cu2+ slowly, thereby reducing its toxicity.
In light of the biomass, photosynthetic pigment, antioxidant enzyme activity, and intracellular structure of lettuce leaves, we conclude that physiological toxicity was higher and the stress response was stronger in RW10 compared with LDH10.
Integrated transcriptome and metabolome analysis of lettuce leaves two weeks after the initial spray
To further investigate the potential molecular mechanism underlying the enhanced growth of lettuce after CuFe-LDH treatment, we conducted a transcriptome analysis of lettuce leaves that had been treated with or without CuFe-LDHs. A total of 770 DEGs (520 up-regulated and 250 down-regulated) were detected in leaves treated with LDH10 relative to the CK (Fig. 4a, Additional file 1: Fig. S4). Similarly, there were 4379 DEGs (2,116 upregulated and 2263 down-regulated) in leaves treated with RW10 relative to the CK (Fig. 4a). The number of up-regulated DEGs was higher than the number of down-regulated DEGs in the LDH10 treatment. Conversely, the number of up-regulated DEGs was lower than the number of down-regulated DEGs in the RW10 treatment (Fig. 4a). The total DEGs of LDH10 and RW10 were clustered into 16 profiles (from profile 0 to 15) based on gene expression patterns using Short Time-series Expression Miner software (Fig. 4b) to identify significantly changed DEGs. The most represented clusters were profiles 0, 2, 3, 7, 8, 12, 13, and 15 (p < 0.01). To gain further insights into transcriptional changes, KEGG enrichment analysis was performed for genes belonging to profiles 0, 2, 3, 7, 8, 12, 13, and 15 (Fig. 4c).
Transcriptome analysis of lettuce leaves after 2 weeks of initial treatment. a Volcano plots depicting the differentially expressed genes (DEGs) with a false discovery rate (FDR) below 0.05 and an absolute fold change of ≥ 2 between various treatment groups (LDH10/CK, RW10/CK, LDH10/RW10). b The expression patterns of DEGs across LDH10 and RW10 treatments were inferred using Short Time-series Expression Miner (STEM) analysis. Each frame represents the expression pattern of all the DEGs, which are indicated by the colored lines. c The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed significantly overrepresented profiles of differentially expressed genes in lettuce leaves in the LDH10 and RW10 treatments. The values are presented as the mean ± standard deviation of three replicates for each treatment. The abbreviations used are the same as in Fig. 2
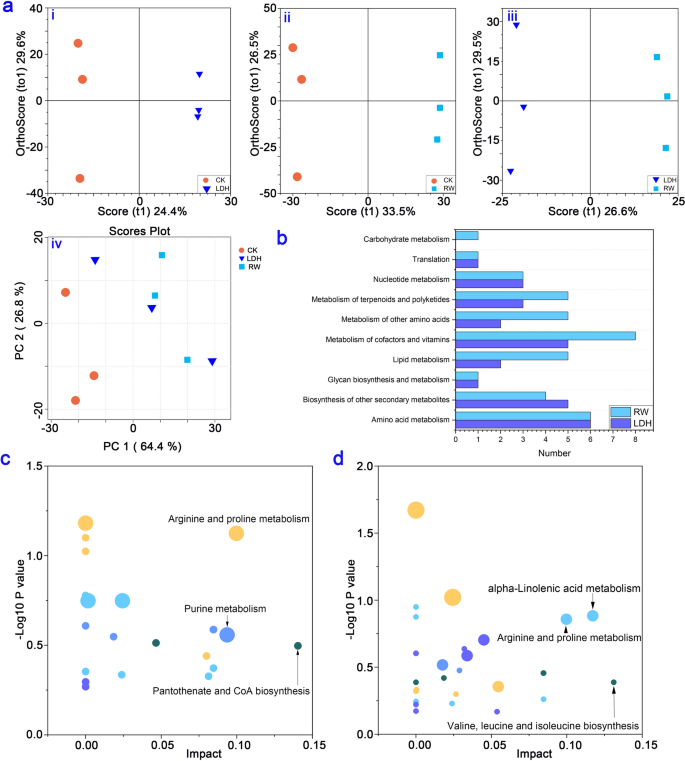
By selecting MS peaks with orthogonal partial least-squares discriminant analysis (OPLS-DA) (VIP > 1, p < 0.05), the total numbers of differential metabolites in each treatment are shown in Additional file 1: Fig. S5. By employing principal component analysis (PCA) and OPLS-DA, we conducted an in-depth analysis of the distinctive metabolic changes stemming from LDH10 and RW10 exposure. The PCA score plot was used to assess the overall effect of LDH10 and RW10 on lettuce leaf metabolites (Fig. 5a). The sampling points corresponding to the three different treatments were noticeably scattered, indicating a substantial effect of LDH10 and RW10 on the metabolites of lettuce leaves. Notably, the effects of LDH10 and RW10 on the metabolic profiles differed significantly (Fig. 5a). Several metabolic pathways significantly differed following LDH10 or RW10 treatment relative to the control group. These pathways include carbohydrate metabolism, translation, nucleotide metabolism, metabolism of terpenoids and polyketides, metabolism of other amino acids, metabolism of cofactors and vitamins, lipid metabolism, glycan biosynthesis and metabolism, amino acid metabolism, and biosynthesis of other secondary metabolites (Fig. 5b). These findings highlight the prominent changes in various key metabolic processes as a result of LDH10 or RW10 treatment relative to the control group. The differential metabolites were analyzed using the Fisher tool to investigate changes in metabolic pathways. Comparative analysis revealed significant effects of the LDH10 or RW10 treatments on specific metabolic pathways compared with the CK (Fig. 5c, d). LDH10 treatment affected arginine and proline metabolism, purine metabolism, and pantothenate and CoA biosynthesis (Fig. 5c). RW10 treatment affected valine, leucine and isoleucine biosynthesis, arginine and proline metabolism, and alpha-linolenic acid metabolism (Fig. 5d). Arginine and proline metabolism was enriched in the leaves of all treatment groups, suggesting that it plays a key role in the response to both LDH10 and RW10 exposure.
Metabolome analysis of lettuce leaves after 2 weeks of initial treatment. a Principal component analysis (PCA) plot and orthogonal partial least-squares discriminant analysis (OPLS-DA) model for identifying differential metabolites in the control and the LDH10 and RW10 treatments. LDH/CK (i), RW/CK (ii), LDH/RW (iii), and PCA (iv). The shape and color of the points correspond to different experimental groups. PC1: first principal component score; PC2: orthogonal principal component score; t1: first principal component score. b Annotation categories of the identified metabolites according to KEGG pathway analysis. Metabolic pathway analysis of the differential metabolites in the treatments of LDH10 c and RW10 d compared with the CK. The abscissa coordinate represents the value of the metabolic pathway. The bubble size indicates the number of metabolites. The vertical coordinate and bubble color indicate the p-value of the enrichment analysis. The abbreviations used are the same as in Fig. 2
Changes in gene expression are one of the various ways in which plants respond to external stimuli [59]. The environmental stress induced by the RW10 treatment was stronger than that induced by the LDH10 treatment, as indicated by the fact that a higher number of functional DEGs were detected in the RW10 treatment than in the LDH10 treatment (Fig. 4a, Additional file 1: Fig. S4). The regulation of gene expression led to changes in the metabolite profiles in lettuce leaves. The number of differential metabolites in lettuce leaves was higher in the RW10 treatment than in the LDH10 treatment (Additional file 1: Fig. S5). Moreover, the metabolic functions of lettuce leaves were significantly affected, especially under RW10-induced stress (Fig. 5). Both RW10 and LDH10 treatments led to the regulation of stress response-related DEGs in plants, including pathways such as plant-pathogen interaction and MAPK signaling pathway-plant (Fig. 4c). The expression of the genes associated with these pathways was up-regulated in profile 15 (Fig. 4c) following RW10 or LDH10 treatment, indicating that LDH10 potentially enhances the resilience of plants to stress. Similarly, Claudia Jonak et al. exposed the seedlings of Medicago plants to excessive copper or cadmium ions and found that they could activate the MAPK cascade reaction of higher plants, thereby reducing their toxicity [60]. The expression of genes related to mechanisms involved in photosynthesis, including the citrate cycle (TCA cycle), carbon metabolism, and photosynthesis, was up-regulated or down-regulated, suggesting that LDH10 and RW10 might induce imbalances in mechanisms related to photosynthesis (Fig. 4c). Zeatin, a well-known cytokinin plant hormone [61] and a regulator of plant growth [62], was elevated under stress conditions in various plant species. The expression of genes related to zeatin was higher in the LDH10 treatment than in the RW10 treatment, indicating that the up-regulation of zeatin could potentially mediate the response of lettuce to LDH10 (Profile 15, Fig. 4b, c). The expression of the genes in Profile 12, 13, and 15 was up-regulated in the LDH10 and RW10 treatments relative to the CK, and the expression of these genes was significantly up-regulated in the LDH10 treatment relative to the RW10 treatment; these genes are associated with the plant-pathogen interaction and MAPK signaling pathway-plant pathways. The plant-pathogen interaction [63] and MAPK signaling pathway-plant [60] are associated with stress resistance, suggesting that LDH10 may enhance the ability of plants to adapt to stress and induce the expression of stress resistance genes compared with the RW10 treatment. The above data indicate that the stress resistance capacity was higher in the LDH10 treatment than in the RW10 treatment.
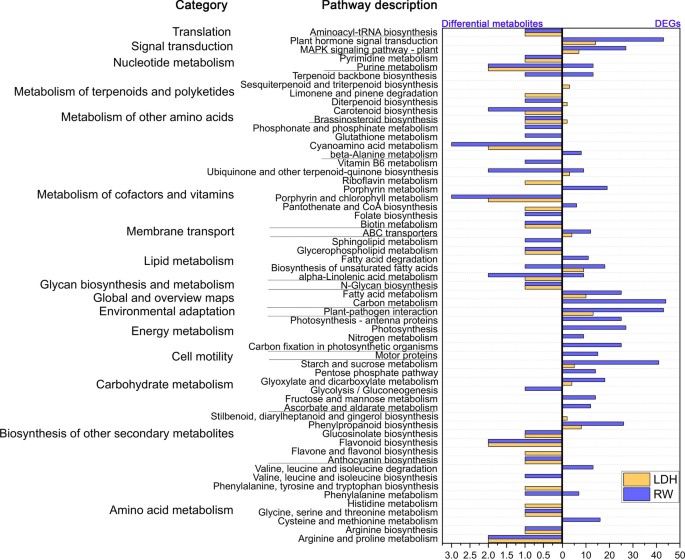
The combined analysis of DEGs and differential metabolites did not reveal shared regulatory mechanisms between LDH10 and RW10 (Fig. 6). RW10 regulated purine metabolism, terpenoid backbone biosynthesis, ubiquinone and other terpenoid-quinone biosynthesis, alpha-linolenic acid metabolism, and biosynthesis of unsaturated fatty acids (Fig. 6). Purine metabolism is a key regulated metabolic pathway in Arabidopsis under drought stress and in rice under spaceflight stress; it also plays a significant role in the ability of rice seedlings to tolerate darkness [64]. The terpenoid backbone biosynthesis pathway and the biosynthesis of ubiquinone and other terpenoid-quinone compounds are responsible for the synthesis of various terpenoids and ubiquinones, which affect multiple physiological functions [65]. Alpha-linolenic acid metabolism and the biosynthesis of unsaturated fatty acids play a role in increasing the content of unsaturated fatty acids to counteract the loss of cellular membrane fluidity induced by adverse conditions [66]. Significantly, LDH10 induced the biosynthesis of Brassinosteroids (BR) (Fig. 6). In light of the known role of BR in promoting growth and enhancing stress resistance [67], LDH10 promoted the expression of genes involved in the biosynthesis of BR, which led to the increased production of BR. These findings regarding the effect on hormonal changes are consistent with previous studies that demonstrated alterations in auxin content and flux in Arabidopsis roots by MgAl-LDHs [16]. Furthermore, the qRT-PCR results (Additional file 1: Fig. S6) revealed that the expression of genes involved in the synthesis of BR was up-regulated following LDH treatment, suggesting that this could be the primary molecular mechanism by which LDH promotes growth.
Integration of DEGs and differential metabolites with KEGG pathway annotations following the LDH10 and RW10 treatments. Abbreviations are the same as in Fig. 2






Add Comment